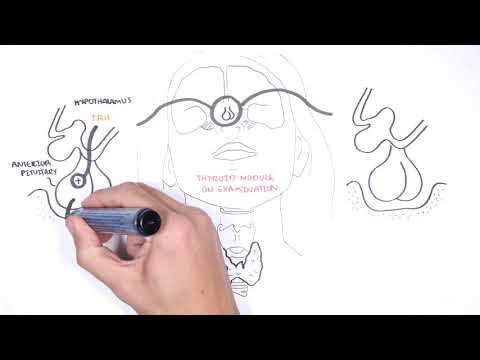
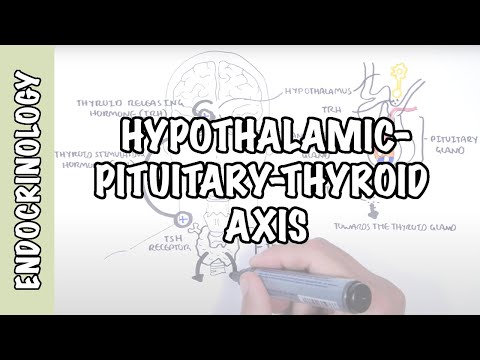
0:00 Hello, in this video we're going to talk about Graves' disease. 0:08 This is an overview and introduction. 0:11 Graves' disease is the most common cause of hyperthyroidism, which is 0:17 essentially the 0:18 thyroid gland working on overdrive producing a lot of thyroid hormones. 0:23 In a Graves' disease is a syndrome that may consist of hyperthyroidism, goiter, 0:29 ophthalmopathy, 0:31 and occasionally dermopathy, which is a skin disease associated with the over 0:36 active thyroid 0:37 gland. 0:39 In order to understand Graves' disease, it is important to learn or recap the 0:44 anatomy 0:45 and physiology of the thyroid gland and its hormones, as well as the hypothal 0:52 amic, 0:52 pituitary, thyroid axis. 0:55 So here is the brain. 0:57 Important structures to note in the brain is the hypothalamus. 1:02 And below the hypothalamus is the pituitary gland, which actually consists of 1:09 two lobes, 1:10 the anterior and posterior lobes, but here we will mainly focus on the anterior 1:17 lobe. 1:17 Here I'm drawing the circulation, which is the blood. 1:21 And the most important organ in the story is the thyroid gland. 1:25 The thyroid gland sits on the trachea, the tracheal rings, right below the l 1:31 arynx. 1:31 And here again is a circulation, the blood. 1:35 So the hypothalamus in the brain secretes thyroid releasing hormone, or TRH, 1:42 which will 1:43 stimulate the anterior pituitary to secrete thyroid stimulating hormone, or TSH 1:51 for short. 1:53 TSH will travel in circulation and target the thyroid gland. 1:59 The thyroid gland has receptors for TSH. 2:03 The binding of TSH to the TSH receptor on the thyroid gland will stimulate the 2:08 thyroid 2:09 gland to produce and secrete thyroid hormones. 2:13 And it will secrete thyroid hormones into circulation. 2:17 These hormones are T3 and T4. 2:22 T3 and T4 are carried in circulation in the blood via proteins, thyroid binding 2:31 protein. 2:32 An increase in T3 and T4 in circulation will have a negative feedback on the 2:37 hypothalamus 2:38 on the brain. 2:39 And the negative feedback will tell the hypothalamus to stop producing TRH and 2:44 thus TSH to reduce 2:47 the thyroid hormone production when we already have enough in circulation. 2:55 So what does T3 and T4 do? 2:57 Well T3 and T4 are lipid soluble. 3:00 And so when they are at the target cell or target organ, they simply detach 3:05 from the 3:06 thyroid binding protein and move inside the cell. 3:10 T4 will get converted to T3 because T3 is the more potent one, you can say. 3:14 It's the more effective one. 3:17 And so T3 is actually also known as triadothyronine, but let's just call it T3. 3:23 T3 will bind onto thyroid hormone receptors within the nucleus where it will 3:28 stimulate 3:29 transcription or it will tell the DNA to make things that will result in 3:35 production of proteins, 3:38 enzymes, all these things that will cause an increase in metabolic rate, 3:43 metabolic activity, 3:45 as well as increased sympathetic activity and growth and development. 3:51 Graves disease is where there is elevation of the thyroid hormones, T3 and T4. 3:57 An increase in T3 and T4 in circulation and the effects of these is called 4:01 hyperthyroidism. 4:02 A Graves disease is not the only cause of hyperthyroidism. 4:08 There are other causes of hyperthyroidism, which include pituitary adenoma, 4:14 which can 4:15 cause more thyroid stimulating hormone, TSH, being produced by the anterior pit 4:21 uitary 4:22 gland. 4:23 More TSH means more stimulation to the thyroid gland to secrete T3 and T4. 4:31 Thyroid medication is also another cause, meaning thyroid hormone analogs can 4:36 of course 4:37 cause hyperthyroidism. 4:40 Iodine and amiodarone can cause or induce hyperthyroidism. 4:46 Thyroid adenoma, specifically toxic thyroid adenoma can cause more thyroid 4:51 hormones being 4:52 produced, which then will cause hyperthyroidism. 4:57 Similarly, toxic multinodular goita can cause hyperthyroidism. 5:03 It's really important to understand that the use of the word toxic really means 5:08 that it's 5:09 cause more things are being produced, and thus it's more toxic. 5:15 So toxic, in this case, means more overproduction of thyroid hormones. 5:23 Have the physiology of Graves disease. 5:26 Again, here is the brain, here we have the hypothalamus and the anterior pituit 5:32 ary gland. 5:33 Here is the thyroid gland and the circulation. 5:36 On the thyroid gland we have the receptor, the TSH receptor. 5:42 In Graves disease there are O2 antibodies being produced. 5:46 What I mean by O2 antibody are antibodies against ourself. 5:51 The main O2 antibodies are the antibodies which basically mimic thyroid 5:56 stimulating hormone. 5:59 So these antibodies will target thyroid stimulating hormone receptors. 6:05 The binding of the thyroid stimulating hormone receptors by these O2 antibodies 6:13 will actually 6:13 stimulate the thyroid gland to produce more thyroid hormones, T3 and T4. 6:21 The increase in T3 and T4, of course, will cause a negative feedback to the 6:26 hypothalamus 6:27 to tell it to stop making TRH and TSH, because we already have enough T3 and T4 6:39 . 6:39 But regardless of the decrease in TSH, the thyroid stimulating hormone receptor 6:45 O2 antibodies 6:46 will still exist, and so we'll keep telling the thyroid gland to produce more 6:51 thyroid hormones. 6:54 This means that there are a lot of thyroid hormones in circulation, and so 6:58 there's overactivity 7:00 of the thyroid hormones causing hyperthyroidism. 7:07 In Graves disease the main antibodies are the thyroid stimulating hormone 7:11 receptor O2 antibodies. 7:12 However, there are other antibodies such as thyroid globulin O2 antibodies and 7:18 thyroid 7:19 peroxidase antibodies or TPO antibodies, but these are less common in Graves 7:25 disease. 7:26 On a side note, thyroid peroxidase antibodies are more often seen in hypothy 7:32 roidism, particularly 7:34 Hashimoto's disease. 7:37 The next question to ask is where do these antibodies come from? 7:41 Well, like most autoimmune diseases, there is no exact pinpoint cause. 7:47 Rather, it is postulated that many factors cause inappropriate or abnormal 7:52 activation 7:53 of immune cells against the thyroid gland. 7:56 Within the lymph nodes, cells called antigen-presenting cells normally activate 8:03 naive T-cells in situations 8:06 where there is an infection or when the body is in trouble. 8:10 The antigen-presenting cell in this scenario may present thyroid stimulating 8:17 hormone receptor 8:18 like antigen, and this will activate the naive T-cell within the lymph node. 8:24 The activated T-cell can then activate B-cells. 8:30 The T-cells will tell the B-cells to become another cell called a plasma cell, 8:36 and it 8:37 becomes a plasma cell, so that it can produce antibodies against the antigen. 8:44 Plasma cells are the cells that secrete and produce antibodies, and so in this 8:50 scenario, 8:51 thyroid stimulating hormone receptor O2 antibodies are produced, but instead of 8:58 damaging the 8:59 thyroid stimulating hormone receptor, it actually stimulates it and acts 9:03 similar to a thyroid 9:05 stimulating hormone. 9:08 The thyroid stimulating hormone receptor O2 antibody produced are not very 9:13 specific, 9:14 meaning that they most often bind to thyroid stimulating hormone receptor, but 9:20 there are 9:20 thyroid stimulating hormone-like receptors all around our body, particularly 9:26 the eyes 9:27 and the legs. 9:29 And so the thyroid stimulating hormone receptor O2 antibodies can cross-react 9:34 with other 9:34 parts of the body, such as it can cross-react with things in the eyes leading 9:40 to ophthalmopathy, 9:42 or cross-react with skin-causing dromopathy. 9:48 Pathology of Graves' Disease After some time, there are some notable 9:52 changes seen in the thyroid gland of patients with Graves' disease. 9:57 So normally, the thyroid gland are made up of follicular cells, which form the 10:02 inner colloid, 10:04 where thyroid hormones are produced. 10:07 In Graves' disease, the follicular cells become packed and squeeze together, 10:14 becoming 10:14 tall cells. 10:17 This then subsequently causes a scant colloid. 10:21 Further, in Graves' disease, there are presence of lymphocytes, so there's 10:26 lymphocytic infiltration. 10:30 The risk factors for Graves' disease include female gender, family history, 10:39 infection leading 10:40 to thyroiditis, which is inflammation of the thyroid gland, stress is also risk 10:47 factor, 10:48 as well as smoking and amiodarone. 10:51 Now remember that Graves' disease is hyperthyroidism and is characterized by 10:56 increase in metabolic 10:57 rate and increase in sympathetic activity. 11:02 And so now let us look at the signs and symptoms of Graves' disease. 11:06 The clinical presentation can include hyperactivity, irritability, insomnia, 11:15 sweating, heat intolerance, 11:19 fatigue, weakness, Graves' ophthalmopathy, feeling thirsty, dysnia, palpit 11:29 ations, weight 11:31 loss, oligomanorrhea, amenorrhea, decreased libido, and symptoms of Graves' 11:42 germopathy. 11:44 All examination or clinical findings can include patient being anxious and 11:50 irritable, presence 11:52 of goita, ophthalmopathy, hair loss, presence of congestive heart failure, t 12:01 achycardia or 12:03 atrial fibrillation, patient might have a fine tremor that can be increased in 12:08 bowel 12:09 sounds, that can be oncolysis, essentially clubbing, hyperreflexia, and also 12:17 presence 12:18 of Graves' germopathy. 12:21 The investigations with someone with suspected Graves' disease. 12:26 Again, just recapping. 12:29 Here's the brain, the hypothalamus, and the anterior pituitary gland which 12:34 produces thyroid 12:35 stimulating hormone or TSH. 12:38 The thyroid gland has the thyroid stimulating hormone receptor. 12:43 In Graves' disease, there are presence of antibodies, otoantibodies. 12:49 So an investigation is to check serum thyroid stimulating hormone which will 12:55 show a decrease. 12:57 Bloods may also show presence of thyroid stimulating hormone receptor otoantib 13:02 odies. 13:03 Thyroid investigation is a thyroid ultrasound which can look at the thyroid 13:07 architecture 13:08 and structure to see any signs of other causes of hyperthyroidism such as toxic 13:14 thyroid adenoma 13:16 or toxic multinodular goita. 13:20 The most important investigation is to check thyroid function which will show 13:25 an increase 13:25 in T3 and T4 levels. 13:29 A thyroid scan such as sin to scanning is an investigation where iodine dye is 13:37 injected 13:38 into the bloodstream. 13:41 In a normal thyroid, iodine is taken up by the thyroid gland and so we can see 13:46 distribution 13:47 of iodine uptake in the thyroid because the iodine is tagged. 13:52 However, in Graves' disease there is a big increase in iodine uptake by the 13:58 thyroid 13:59 gland because the thyroid gland is working on overdrive. 14:03 It is producing a lot of hormones. 14:08 Management The management of Graves' disease can be 14:10 divided into pharmacological, radioidine therapy and surgery. 14:18 Medical or pharmaceutical therapy include the use of anti-thyroid drugs such as 14:23 theomide. 14:24 The mechanism of action of theomide basically inhibits the enzyme thyroid perox 14:30 idase which 14:30 normally helps in the synthesis of T3 and T4. 14:35 And so inhibiting thyroid peroxidase or TPO will decrease T3 and T4 thyroid 14:43 hormone levels. 14:47 Beta blockers is also another form of pharmacological management and the 14:52 mechanism of action is 14:53 to decrease the sympathetic activity by blocking the beta adrenergic receptors. 15:00 Beta blockers are given because we see signs of heightened sympathetic activity 15:04 in Graves' 15:05 disease such as tachycardia. 15:09 Radioidine therapy is the second management for Graves' disease and it is used 15:14 for people 15:15 who don't want to take medication or want something alternative than surgery or 15:22 medication. 15:23 Essentially in radioidine therapy a radioactive iodine is taken either via 15:29 liquid or pill. 15:31 The iodine taken is radioactive and so naturally decays to xenon. 15:36 When it decays it emits energy which theoretically destroys the surrounding 15:43 thyroid tissue. 15:44 Thus the destroyed thyroid tissue will decrease thyroid hormone production. 15:50 Surgery is the third type of management for Graves' disease and for people who 15:55 do not 15:56 want radioidine therapy and where medication is not useful or is ineffective. 16:03 The surgery is thyroidectomy which is removal of the thyroid gland and this can 16:09 be partial 16:10 or total thyroidectomy. 16:14 Temperatures of Graves' disease include congestive heart failure, atrial fibr 16:20 illation, decrease 16:21 in bone density leading to osteoporosis, Graves' ophthalmopathy complications 16:28 include 16:29 lary vision and Graves' dormopathy. 16:34 The complication of this in severe cases is elephantitis dormopathy. 16:40 Finally, it is important to know the complications associated with thyroid 16:46 ectomy such as allaceration 16:48 to the laryngeal nerves, internal bleeding, infection post surgery and 16:54 accidentally removing 16:56 the parathyroid gland which actually sits on the thyroid gland.