Granulomatosis with Polyangiitis
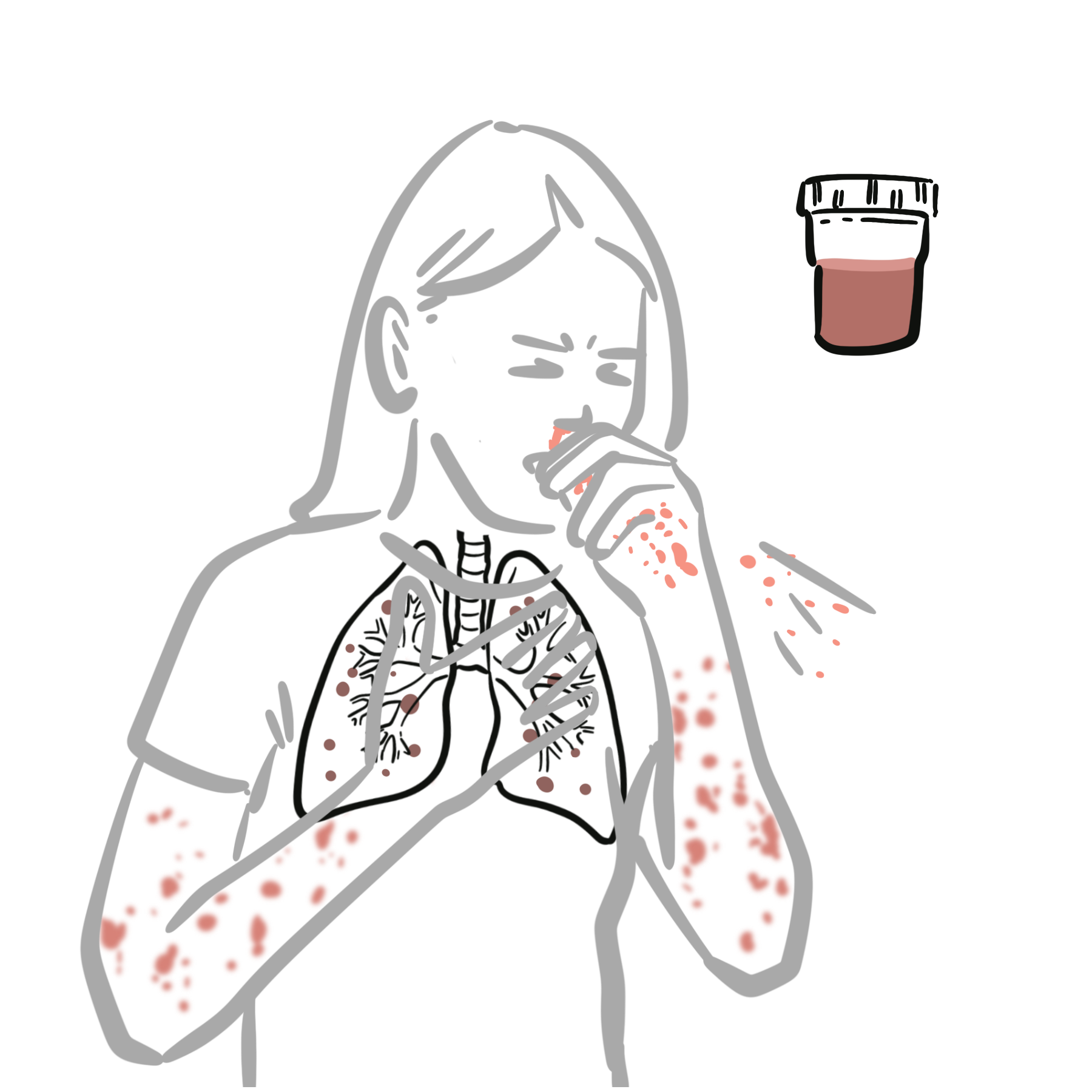

Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis) is a rare, chronic, systemic necrotising vasculitis affecting small-to-medium-sized vessels, characterised by granulomatous inflammation, necrotising vasculitis, and tissue destruction, primarily involving the upper and lower respiratory tracts and kidneys. It falls under the ANCA-associated vasculitides (AAV). Peak incidence is in the 5th–6th decade, with slight male predominance and higher prevalence in Caucasians.
Vasculitis: Inflammation of blood vessels, causing vessel wall damage and downstream ischemia or haemorrhage.
Granuloma: Organised collection of macrophages in response to persistent inflammation.
ANCA (anti-neutrophil cytoplasmic antibodies): Autoantibodies targeting neutrophil cytoplasmic components; associated with GPA and other AAVs.
Necrotising glomerulonephritis: Rapidly progressive kidney inflammation with crescent formation, common in GPA.
Upper airways (sinuses, nasal mucosa): Rich vascular network vulnerable to necrotising inflammation → septal perforation
Lower respiratory tract (lungs): Bronchi and alveoli susceptible to granulomatous inflammation and cavitating nodules
Kidneys: Glomeruli affected by pauci-immune crescentic glomerulonephritis → rapid renal failure
Immune system: Neutrophil activation by ANCAs → release of lytic enzymes and oxygen radicals → endothelial damage
Aetiology
Unknown, likely multifactorial
Risk Factors
Chronic nasal carriage of Staph aureus is associated with GPA relapses【1】.
GPA uniquely combines vasculitis and granulomatous inflammation, distinguishing it from other AAVs.
If a patient with presumed asthma does not respond to treatment and has stridor or biphasic wheeze, think GPA with subglottic stenosis—especially if they have ENT symptoms or PR3-ANCA positivity.
Classification Criteria for GPA, MPA, and EGPA (2022)
| Entry Requirement: A diagnosis of small- or medium-vessel vasculitis has been made (biopsy), and other mimicking conditions have been excluded. | |||
| Variables | GPA | MPA | EGPA |
| Clinical criteria | |||
| Nasal passage involvement | +3 | −3 | — |
| Cartilaginous involvement | +2 | — | — |
| Conductive or sensorineural hearing loss | +1 | — | — |
| Obstructive airway disease | — | — | +3 |
| Nasal polyp | — | — | +3 |
| Mononeuritis multiplex | — | — | +1 |
| Laboratory criteria | |||
| PR3-ANCA (or C-ANCA) positivity | +5 | −1 | −3 |
| MPO-ANCA (or P-ANCA) positivity | −1 | +6 | — |
| Serum eosinophil ≥1000/µL | −4 | −4 | +5 |
| Hematuria | — | — | −1 |
| Histological criteria | |||
| Granuloma, granulomatous inflammation, or giant cells | +2 | — | — |
| Pauci-immune glomerulonephritis | +1 | +3 | — |
| Extravascular eosinophilic-predominant inflammation | — | — | +2 |
| Radiological criteria | |||
| Pulmonary nodules, mass, or cavitation on chest imaging | +2 | — | — |
| Fibrosis or ILD on chest imaging | — | +3 | — |
| Nasal/paranasal sinusitis or mastoiditis on imaging | +1 | — | +1 |
| Total Score Cut-off for Classification | ≥5 | ≥5 | ≥6 |
PR3‑ANCA positivity raises the likelihood of GPA and is associated with a higher risk of relapse than MPO‑ANCA positivity.
ANCA serology:
A positive c-ANCA (PR3) in a patient with upper/lower respiratory tract symptoms and haematuria is highly suggestive of GPA.
Induction therapy (for active/severe disease):
Maintenance therapy (after induction):
Adjuncts
Plasma exchange may be considered in patients with rapidly progressive glomerulonephritis or diffuse alveolar hemorrhage.
Complications
Poor prognostic factors
Prognosis

Please confirm you want to block this member.
You will no longer be able to:
Please allow a few minutes for this process to complete.
Discussion