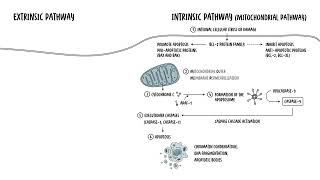
0:00 Appoptosis or program cell death is a tightly regulated process that eliminates 0:18 damaged, 0:19 dysfunctional or unnecessary cells without causing inflammation. 0:25 This ensures tissue homeostasis, preventing the accumulation of potentially 0:31 harmful and 0:32 defective cells. 0:33 It is also a key defense mechanism against certain diseases as it removes cells 0:39 with 0:40 irreparable DNA damage, reducing the risk of cancers and destroys also infected 0:46 cells 0:46 to limit the spread of the infection. 0:55 So let's delve into the mechanism of apoptosis. 0:58 How does it occur? 0:59 Well apoptosis is mediated through two main pathways, the intrinsic or extrins 1:07 ic pathway. 1:09 Both pathways will culminate in the activation of cast bases, the enzymes 1:15 responsible for 1:17 dismantling the cell. 1:24 So let's focus on the intrinsic pathway first, which is also known as the 1:28 mitochondrial pathway. 1:31 The intrinsic pathway is triggered by internal, cellular stress or damage. 1:37 This can include DNA damage, oxidative stress or nutrient deprivation. 1:42 This pathway centers around the mitochondria, hence also known as a 1:47 mitochondrial pathway. 1:49 So here are the steps involved in summary. 1:51 Firstly, you have a triggering event, the internal stimuli such as DNA damage, 1:57 hypoxia or oncogene 1:59 activation. 2:00 This will activate the intrinsic pathway, which involves step two. 2:05 The mitochondrial outer membrane permeabilization or MOMP. 2:11 So the stress signals that the trigger will activate a group of family of 2:17 proteins called 2:18 the BCL2 family. 2:20 The BCL2 family protein consists of members that either promote or inhibit 2:26 apoptosis. 2:28 And they control apoptosis by governing the mitochondrial outer membrane perme 2:33 abilization, 2:34 which is a key step in the intrinsic pathway of apoptosis. 2:39 So the pro-apoptotic proteins, these are the ones that promote apoptosis 2:43 include backs 2:44 and back proteins. 2:47 They cause a mitochondria to release something called cytochrome C. 2:51 Then you have the anti-apoptotic proteins, the BCL2 or the BCL-XL. 2:56 These counteracts the actual backs and back proteins, but if the pro-apoptotic 3:04 protein 3:04 signals dominate, so the backs and the back they dominate, the mitochondrial 3:10 membrane 3:11 will become permeable, hence the mitochondrial outer membrane permeabilization. 3:18 And when this occurs, you have step three where the mitochondria releases cyto 3:23 chrome C. 3:25 The permeabilization of the mitochondria allows cytochrome C to escape into the 3:31 cytosol. 3:32 And once in the cytosol, cytochrome C helps to form the apoptosome. 3:40 Cytochrome C binds to APA-F1, which is the apoptotic protease activating factor 3:46 one. 3:47 This complex that will then recruit pro-caspase-9 forming the apoptosome. 3:54 Caspase-9 is activated within the apoptosome, and essentially what you get is 4:00 this cascade 4:00 of activation of caspase-9. 4:05 Caspase-9 will then activate the executioner caspases other enzymes, which 4:10 include caspase-3 4:12 and caspase-7. 4:14 These enzymes are part of the executioner caspases and are responsible for 4:20 cellular 4:21 dismantling. Essentially, these executioner caspases, these enzymes that are 4:27 activated 4:28 will cleave structural and regulatory proteins, which will lead to chromatin 4:33 condensation, 4:34 DNA fragmentation, and the formation of the apoptotic bodies. 4:40 So I hope that makes sense, the intrinsic pathway which leads to the execution 4:44 er pathway 4:45 and the caspases involved in apoptosis. 4:48 Then you have the extrinsic pathway, which is also known as a death receptor 4:52 pathway. 4:53 Now, the extrinsic pathway is activated by external signals, hence extrinsic. 4:59 Typically, 4:59 it involves ligands binding onto the death receptor on the cell surface. So let 5:03 's look 5:03 at the key steps involved. 5:05 Number one, you have the triggering event. The extrinsic pathway triggers apopt 5:10 osis in 5:10 response to an external stimuli, namely a binding of a ligand binding to a 5:15 death receptor 5:16 on the cell surface. And these receptors are typically members of the tumor nec 5:20 rosis factor, 5:21 receptor gene family, such as TNF receptor 1 or FAS. Binding at these receptors 5:28 will lead 5:28 to downstream activation of caspase enzymes. 5:32 Step number two, you have what's called formation of the death-inducing signal 5:37 complex or disk. 5:40 So when the ligand binds to the death receptor, it actually forms a trimeric 5:46 structure and 5:47 recruits what's called adaptor proteins such as FADD or FAS associate death 5:54 domain proteins. 5:56 Essentially, what this adaptor protein such as FADD will do, it will then 6:02 recruit procaspase, 6:05 specifically procaspase-8. And this all is what's known as the death-inducing 6:12 signal complex. 6:15 Once this occurs, you get an activation of caspase-8, but as well as caspase-10 6:22 . Within 6:23 the death-inducing signal complex, the procaspase-8 essentially becomes cleaved 6:30 . Then you have 6:31 step three where the death-inducing signal complex will activate the procasp 6:36 ases, caspase-8, 6:38 as well as another one, caspase-10. Essentially, what happens is procaspase-8 6:45 and procaspase-10 6:47 will become cleaved and activated into caspase-8 and caspase-10. This then 6:53 continues and you 6:54 get a caspase-cascade activation. Caspase-8 and caspase-10 will then directly 7:02 activate 7:02 the executioner caspases, caspase-3 and caspase-7, two, dismantle the cell as 7:09 we talked about 7:10 earlier. Importantly though, in step four here, there is actually a cross-talk 7:15 with the intrinsic 7:16 pathway. In some cases, caspase-8 can cleave something called BID, a pro-appopt 7:23 otic BCL2. 7:24 Now truncated BID translocates to the mitochondria promoting the release of cy 7:35 tochrome C, which 7:37 will then amplify apoptosis through the intrinsic pathway. Anyway, going back 7:43 to the extrinsic 7:44 pathway, you get these executioner caspases which are activated through the c 7:48 aspase-cascade 7:49 activation, and these executioner caspases-3 and caspase-7 will then cause cle 7:55 avage or cellular 7:56 proteins leading to apoptotic body formation, and essentially you get what's 8:02 called apoptosis. 8:03 These apoptotic bodies will then be phagocytized by surrounding cells and mac 8:08 rophages. So I 8:10 hope this diagram makes sense, and essentially important to note that the 8:15 common endpoint 8:16 between the intrinsic and extrinsic pathway is the executioner caspases. Both 8:27 pathways 8:28 converge on the activation of executioner caspases-3 and caspase-7, which 8:34 orchestrates 8:35 the dismantling of the cell by fragmenting the DNA, degrading nuclear and cytos 8:41 keletal 8:42 proteins and forming the apoptotic bodies for safe removal by phagocytes. 8:53 So what do you actually see morphologically in apoptosis? Well, again, once the 8:58 executioner 8:59 caspases are active, the following morphology occurs in the cell. Firstly, the 9:06 cell shrinks. 9:08 When the cell decreases in size, the cytoplasm becomes dense, and the organell 9:12 es are more 9:13 tightly packed. Then you get chromatin condensation. Chromatin aggregates at 9:22 the nuclear periphery. 9:24 The dense masses of chromatin can be seen under electron microscopy. This 9:28 process leads 9:29 to nuclear fragmentation, step 3. Nuclear fragmentation is where the nucleus 9:38 progressively condenses 9:39 and breaks up. These fragments are often dispersed in the cytoplasm. Then you 9:49 get membrane blebbing. 9:50 The plasma membrane shows protrusion called blebs. These blebs contain portions 9:56 of the 9:57 cytoplasm and even organelles. Finally, you get formation of the apoptotic 10:04 bodies. The cell breaks apart into membrane-bound vesicles known as apoptotic 10:09 bodies. These contain 10:11 cytoplasm, organelles and nucleofragmins. The apoptotic bodies are rapidly 10:16 recognized 10:16 and phagocytized by neighboring cells or macrophages. Without triggering 10:23 inflammation, 10:24 apoptotic cells lose their connection with the neighboring cells, and this 10:27 contributes 10:27 to the detachment of the apoptotic cells from its tissue environment. Through 10:33 apoptosis, 10:33 the plasma membranes actually remain intact, maintaining the cell's compartment 10:38 alization. 10:38 This prevents the leakage of the actual cellular components, avoiding an 10:45 inflammatory response. 10:47 As mentioned, the apoptotic bodies are rapidly phagocytized by neighboring 10:52 cells, including 10:53 macrophages. Apoptotic bodies can be recognized inside these cells, but 10:58 eventually they become 10:59 degraded. If the fragmented cells are not phagocytized, it will undergo 11:04 degradation, 11:05 which resembles necrosis in a process called secondary necrosis. 11:15 So in summary, apoptosis is a vital biological process that plays a critical 11:19 role in maintaining 11:20 the health and balance of multicellular organisms. It is essential for normal 11:26 development, immune 11:27 system function and tissue homeostasis. Apoptosis is crucial for removing 11:32 damaged or mutated 11:34 cells that could otherwise lead to cancers or other diseases. Unlike necrosis, 11:39 apoptosis 11:40 is a controlled process that prevents inflammation by neatly packaging cellular 11:46 debris into apoptotic 11:47 bodies for phagocytosis.