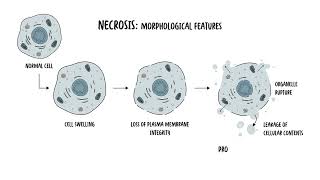
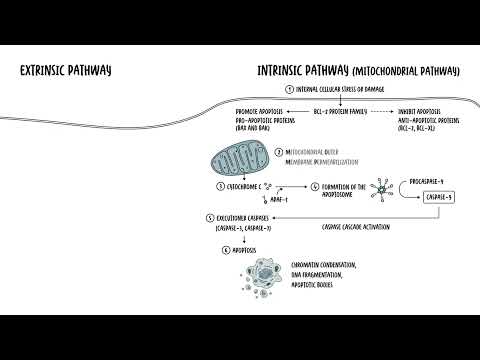
0:00 Cell death is a fundamental biological process crucial for maintaining cell hom 0:17 ostasis development 0:19 and tissue integrity. 0:21 There are two main types. 0:23 These are apoptosis, or programmed cell death, and necrosis, which is 0:29 uncontrolled cell death. 0:33 Apoptosis is a tightly regulated process that eliminates damage, dysfunctional, 0:38 or 0:38 unnecessary cells without causing inflammation, whereas necrosis is an 0:45 uncontrolled form of 0:47 cell death, resulting from sudden injury, such as trauma infection or schemia. 0:53 It causes or triggers inflammation. 1:02 Let's take a look at apoptosis first, beginning with the mechanism. 1:09 So apoptosis is mediated through two main pathways, the intrinsic and extrinsic 1:17 pathway. 1:18 Both pathways culminate in the activation of executioner cast spaces, the 1:24 enzymes responsible 1:25 for dismantling the cell, the cell that's about to die. 1:32 So you have two main pathways. 1:34 The intrinsic pathway is the mitochondrial pathway. 1:38 It's triggered by internal stress signals, such as DNA damage or oxidative 1:43 stress. 1:43 It involves the release of cytochrome C from the mitochondria, activating 1:47 eventually cast 1:48 space 9, a specific enzyme. 1:51 This leads then to the activation of the executioner cast spaces, that 1:55 dismantle cellular components. 2:00 Then there is the extrinsic pathway of apoptosis, which is also known as the 2:04 death receptor pathway. 2:06 This is initiated by an external signal via death receptors, such as FAS or TNF 2:13 receptors. 2:14 Binding of a ligand to these receptors activates cast space 8 and 10, leading 2:20 then to the activation 2:22 of the executioner cast spaces. 2:25 So both the intrinsic and extrinsic pathway of apoptosis will converge on the 2:31 activation 2:32 of the executioner cast spaces, which are enzymes. 2:41 These executioner cast spaces are cast space 3 and cast space 7, which orche 2:47 strate the dismantling 2:48 of the cell, which orchestrate apoptosis. 2:52 Now the dismantling of the cell occurs by fragmenting the DNA, degrading 2:58 nuclear and cytoskeleton 3:00 proteins and forming apoptotic bodies for safe removal by phagocytes. 3:11 So the morphological features, or what we see macroscopically when apoptosis 3:17 occurs, 3:18 is first of all, you get cell shrinkage, then chromatin condensation and 3:25 nuclear fragmentation, 3:28 followed by formation of these apoptotic bodies, which are then phagostized by 3:33 neighboring 3:34 cells, by the macrophages, and so this will not elicit any crazy inflammation. 3:42 It's very programmed. 3:49 As mentioned, apoptosis is important to maintain homeostasis. 3:53 For example, it's important in embryonic development, such as the digit 3:57 formation. 3:58 It's important in eliminating autoreactive T cells in the thymus, because you 4:02 don't 4:02 want any T cells that are autoreactive, because these can then cause autoimmune 4:08 problems. 4:09 Apoptosis is also important for removal of aged or damaged cells, such as sen 4:13 escent or 4:14 infected cells. 4:21 So apoptosis is programmed cell death. 4:24 Then you have necrosis, which is uncontrolled cell death. 4:27 Remember, necrosis is typically a result of acute injury, such as trauma, 4:34 infection or 4:35 ischemia. 4:36 Unlike apoptosis, necrosis often triggers inflammation due to the release of 4:42 cellular 4:43 components into the space, the extracellular space, and this will trigger 4:51 inflammation. 4:53 So what is a mechanism of necrosis? 4:55 Well, necrosis is typically associated with, again, severe cellular damage that 5:01 overwhelms 5:02 the cell's repair mechanism. 5:04 Common triggers include ischemia and hypoxia, lack of oxygen and nutrients, 5:09 toxins and infections, 5:11 such as bacterial toxins or viral psychopathic effects, and of course, trauma, 5:16 like physical 5:17 damage to the actual tissue, the cell. 5:25 So what happens morphologically here? 5:28 Well, in necrosis, the actual cell swells up. 5:32 They lose then their plasma membrane integrity, and the organelles start rupt 5:40 uring. 5:41 Because of the loss of plasma membrane integrity, there is cellular leakage, 5:46 the leakage of 5:47 cellular contents, and this will then include pro-inflammatory molecules and 5:53 damage-associated 5:55 molecular patterns, and this, in turn, will trigger inflammation. 6:03 Remember, these damage-associated molecular patterns and all these things that 6:07 are spilt 6:07 out of the cell needs to be cleaned up, and because it's all messy, it will 6:11 trigger inflammation. 6:19 So if we compare this necrosis to the morphological change in apoptosis, you 6:26 can see in apoptosis 6:28 the things condense, they get smaller and form apoptotic bodies to help clear 6:33 up the 6:33 mess easier. 6:35 Whereas in necrosis, again, it becomes messy. 6:38 The cell swells up, and then it spills all the contents out, and this will 6:44 trigger inflammation. 6:50 So, the clinical implications of necrosis, again, necrosis often results from 6:56 acute 6:56 injuries like trauma, infection, and ischemia, and this could include things 7:00 like a heart 7:01 attack or a stroke. 7:03 And unlike apoptosis, necrosis triggers inflammation due to the release of the 7:08 cellular contents, 7:10 and this can then the inflammation can then damage surrounding tissues and 7:14 exacerbate 7:15 the injury. 7:16 This inflammatory response contributes to pain, swelling, and further cell 7:20 death, complicating 7:21 recovery and often leading to chronic tissue damage or fibrosis. 7:30 That was apoptosis and necrosis, but funnily enough, there are other forms of 7:40 cell death. 7:42 Aside from apoptosis and necrosis, there are other ways cells can die. 7:47 Pyropetosis and necropetosis are both inflammatory, litic cell death pathways. 7:54 Compared to apoptosis where the dying cell's contents are sealed in apoptotic 7:59 bodies, in 8:00 pyropetosis and necropetosis, the leakage of soluble intracellular contents 8:07 into the 8:08 extracellular space contributes to an inflammatory response. 8:14 So, in necropetosis, this combines features of both apoptosis and necrosis. 8:25 You can call it a programmed necrosis. 8:30 Necropetosis and apoptosis can be triggered by similar upstream signals such as 8:35 the activation 8:36 of the death receptor, remember the TNF receptor, for example, on the cell 8:41 surface. 8:42 Necropetosis is mediated by specific proteins such as RIPK1 and RIPK3. 8:51 Necropetosis serves as a backup mechanism, especially when apoptosis is 8:54 inhibited by pathogens 8:56 ensuring cell death and activating an immune response to contain the infection. 9:06 The other form of inflammatory cell death is pyropetosis, which occurs mainly 9:11 in immune 9:12 cells during infections. 9:14 Pyropetosis involves the activation of gas-derming proteins, which will form 9:19 pores in the cell 9:20 membrane, leading to cell lysis and the release of pro-inflammatory cytokines. 9:30 Other less common ways cell die include otophagic cell death. 9:35 Here, the cells digest their own components in response to extreme stress. 9:42 In apoptosis, the entire cell is systematically dismantled and eliminated. 9:48 While in otophagic, specific organelles or portions of the cytoplasm are 9:54 degraded and 9:55 recycled through the lysosomal pathway with the lysosomes, prolonged or 10:01 excessive otophagic 10:02 can also lead to a type of cell death closely linked to apoptoid pathways. 10:10 Finally, you have feroptosis, which is an iron-dependent cell death driven by 10:18 lipid 10:18 peroxidation and oxidative stress. 10:21 It's distinct from otophagy and apoptosis. 10:25 It features mitochondrial changes, like reduced, cristae, membrane rupture and 10:33 condensation. 10:35 In later stages, the membrane ruptures and may trigger inflammation resembling 10:39 pyropetosis 10:40 and necrosis. 10:47 So in summary, let's compare specifically apoptosis and necrosis. 10:53 The triggers are physiological, and it can be also triggered through a signal, 10:58 such as 10:59 the TNF receptor or the FAST receptor. 11:04 Apoptosis is a programmed cell death and requires energy. 11:08 It's energy-dependent. 11:10 The morphological change includes cell shrinkage and chromatin condensation. 11:16 There is no inflammation. 11:17 It is important in development and homeostasis. 11:22 And necrosis occurs from severe injury and always some form of pathological 11:27 condition. 11:28 It is an uncontrolled and energy-independent process, so it doesn't really need 11:34 energy. 11:35 It just occurs because it's damaged. 11:38 The morphological change is that you get the cell swelling. 11:41 It becomes bigger, and then the membrane ruptures, releasing all the content, 11:45 and so 11:46 causes significant inflammation. 11:50 It's a response to an acute injury.