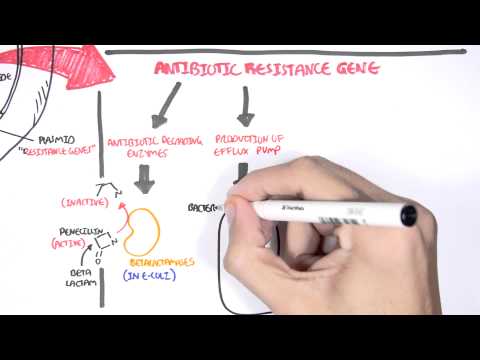
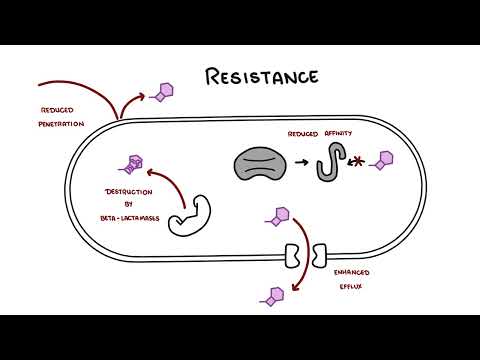
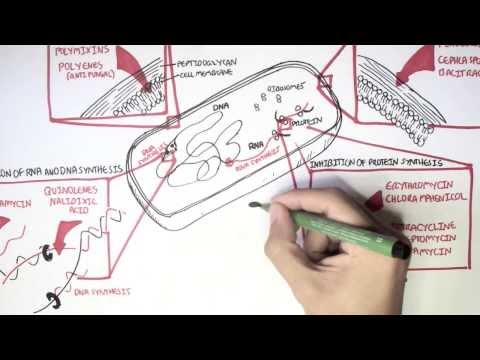
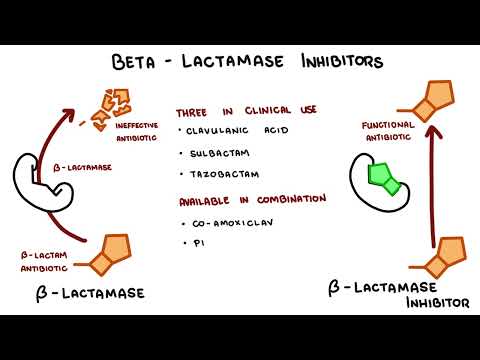
0:00 Hello, in this video, we're going to talk about carbapenums, which are beta-l 0:08 actam antibiotics 0:09 derived from theanomycin, a compound produced by streptomyces catalaya. 0:16 Carbapenums include antibiotics such as imipendum, meropenum, and ertopenum, 0:22 and these guys 0:22 are the hard hitters and used to target a variety of bacteria. 0:29 Carbapenums are a type of beta-lactam antibiotics because of the beta-lactam 0:35 ring. 0:35 Other antibiotic groups that have a beta-lactam ring in their molecular 0:41 structure include 0:41 penicillins, kephlosporins, and monobactams. 0:46 In contrast to penicillins, which we are all aware of, carbapenums have a 0:52 carbon instead 0:54 of a sulfur atom in the first position and an unsaturated bond in the fifth mem 1:00 bered 1:00 a ring. 1:03 Carbapenum, unique side chains in the transposition unlike in the cisposition, 1:08 like in the other 1:08 beta-lactams, make them resistant to several beta-lactamases, which are these 1:14 enzymes 1:15 that essentially inhibit the beta-lactam antibiotics. 1:19 The carbapenums are resistant to several beta-lactamases like extended spectrum 1:28 beta-lactamases. 1:31 So let's talk about the mechanism of action. 1:33 Broadly speaking, as a compound class, carbapenums show limited permeability of 1:39 the outer membrane 1:40 of gram-negative bacteria, and for this reason, carbapenums are reliant on the 1:44 presence of 1:45 an outer membrane protein porent to facilitate their entry into the gram- 1:49 negative bacteria. 1:51 For example, the OPRD is implicated as a key transport channel for carbapenums 1:56 facilitating 1:57 entry into pseudomonas originosa. 2:00 As like other beta-lactam antibiotics, carbapenums affect bacterial cell wall 2:04 and prevent bacterial 2:05 cell wall synthesis, resulting in bacterial cell lysis. 2:11 They show bactericidal properties against both gram-positive and gram-negative 2:17 bacteria. 2:18 Carbapenums bind to penicillin binding proteins, or PBPs, and they essentially 2:24 inactivate them. 2:27 What happens is they prevent the final transpeptidation step during the 2:31 bacterial cell 2:32 wall synthesis, the peptidoglycan synthesis, resulting in no elongation and 2:37 cross-linking 2:38 of the peptidoglycan in the bacterial cell wall. 2:41 And as a result, the bacteria can't develop their cell wall, and ultimately, 2:51 they die. 2:53 But of course, like many antibiotics, resistance can develop. 2:59 And despite being a very powerful beta-lactam, bacteria itself can still 3:04 develop all these 3:05 resistance against the carbapenums. 3:07 And resistance in general, how they develop in beta-lactam antibiotics, occur 3:13 through 3:14 four mechanisms. 3:15 First of all, the production of a low affinity penicillin binding protein 3:22 target; second, 3:23 the reduced outer membrane permeability due to the absence of an outer membrane 3:29 protein, 3:30 such as mentioned the OPRD in gram-negative bacteria. 3:35 The presence of an efflux pump or channel that will allow the drug to be pumped 3:40 out 3:41 of the bacteria, specifically gram-negative bacteria. 3:44 And finally, importantly, the production of beta-lactamases, which are these 3:50 enzymes 3:50 that hydrolyze and break down beta-lactam antibiotics, including carbapenums. 3:56 And in carbapenums case, if they are broken down and become ineffective, these 4:01 enzymes 4:01 that break carbapenums down are called carbapenamases. 4:08 And so I want to talk more about carbapenum resistance, which is very important 4:15 . 4:15 Carbapenums are normally effective against a wide range of bacteria, especially 4:19 a group 4:19 of bacteria that produce ESBL, which refers to extended spectrum beta-lactam 4:26 ases. 4:26 ESBL-producing intero-bacteria group, for example, are a big problem because 4:34 they produce 4:35 a lot of these enzymes, these beta-lactamases, that inhibit the antibiotics 4:41 prescribed, including 4:42 penicillins and kephlosporins. 4:47 Cuz they are actually effective against ESBL-producing bacteria. 4:56 However, carbapenum resistance do occur, and they occur in interobacter group. 5:04 And they are due to a number of combinations of impermeability of the 5:09 antibiotic, by loss 5:11 of membrane porens, for example, plus efflux pump system, plus the presence of 5:16 beta-lactamases. 5:17 Such as still the ESBL or the AMPC enzyme. 5:23 The ESBL and AMPC beta-lactamases, both of which are chromosomally encoded beta 5:32 -lactamase. 5:33 Resistance to carbapenums are scary, as carbapenums are often seen as the top 5:38 dog in antibiotics. 5:39 And thus, appropriate use of antibiotics from the get-go and reducing the risk 5:44 of antimicrobial 5:45 resistance is very important. 5:51 So when do carbapenums get used? 5:54 Carbapenums have the broadest anti-bacterial spectrum of all the beta-lactam 5:59 antibiotics. 5:59 Imipenum is slightly more active against gram-positive bacteria, whereas mer 6:03 openum and 6:04 urtopenum are slightly more active against gram-negative species. 6:09 Meropenum is most active against pseudomonous originosa. 6:14 Infections may be used to treat a wide variety of severe infections, such as 6:18 obstetrics 6:19 and gynecological infections, complicated urinary tract infections, soft tissue 6:24 and 6:24 bone infections. 6:34 Imipenum and meropenum are also used for the treatment of serious infections, 6:38 such as 6:39 patients with polymicrobial infections, febrile neutropenia and nosochomial 6:44 infections, such 6:45 as those caused by pseudomonous originosa or actinobacter species. 6:50 As mentioned, calapenums are also used to treat ESBL-producing gram-negative 6:56 bacterial 6:57 infections. 7:01 Carbapenums are also used for infections caused by the MC-beta-lactamase- 7:06 producing organisms. 7:09 And this group is called the S-CAM group, which include interobacter species, 7:18 seracious 7:20 species, C4ND, actinobacter species, P4garus, providentia species, and Mmogani. 7:33 The S-CAM group is an important one to remember, as this often comes up in 7:36 exams, and again 7:38 is related to the production of MC-beta-lactamase. 7:48 Imipenum and meropenum and eropenum have poor oral absorption and are given 7:53 intravenously. 7:54 Imipenum and meropenum are pharmacologically similar, with a plasma half-life 7:57 of one hour, 7:58 whereas eropenum has a plasma half-life of four hours, which permits a one- 8:03 daily dosing. 8:04 All calapenums are widely distributed and penetrate inflamed meninges. 8:09 All calapenums are renal excreted and require dose modifications and renal 8:14 failure. 8:14 Monitoring the renal function is vital because calapenums should always be dose 8:20 adjusted. 8:20 Very important, when using imipenum, imipenum is actually a substrate for an 8:26 enzyme called 8:27 renal dehydropepidase 1. 8:31 And so, when using imipenum, it's important to co-administer Cilostatin, this D 8:39 HP1 enzyme 8:40 inhibitor, to allow imipenum to still function. 8:50 And this leads to the toxicity and side effects. 8:53 Being a lactam allergic reactions are common side effects. 8:56 A rash or a carrier, immediate hypersensitivity and cross-reactivity with penic 9:01 illums. 9:02 Imipenum causes nausea if infused too quickly and can cause seizures. 9:07 Imipenum, as I mentioned, is actually a substrate for an enzyme, and it's 9:12 broken down in the 9:13 kidneys to a toxic metabolite by the enzyme DHP1. 9:18 And so Cilostatin is used to sustain the body's level of imipenum and to 9:21 prevent the actual 9:23 toxic substrate, which is actually damaging to the kidneys. 9:33 So in summary, copepenums are a beta-lactam antibiotic that are used for a 9:38 variety of infections 9:39 and target both gram-positive but in particular gram-negative bacteria. 9:44 Its unique structure allows it to work against a lot of bacteria, including 9:49 extended spectrum 9:51 and beta-lactamases.