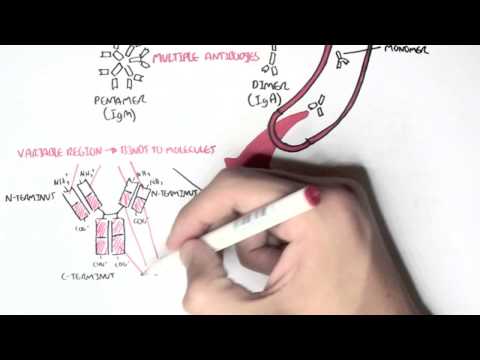
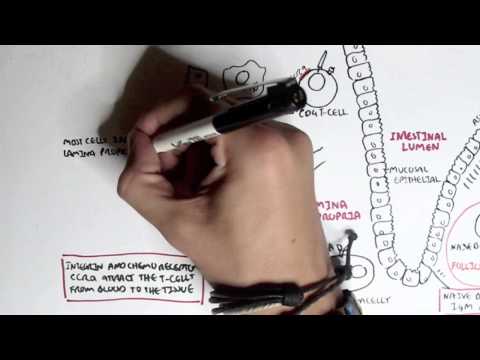
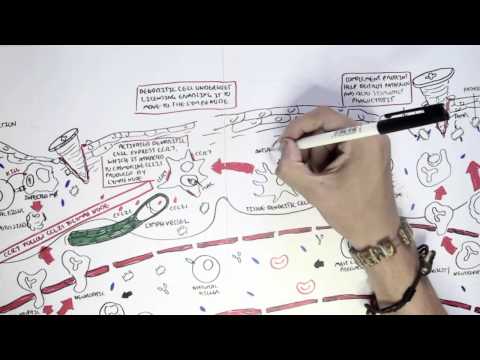
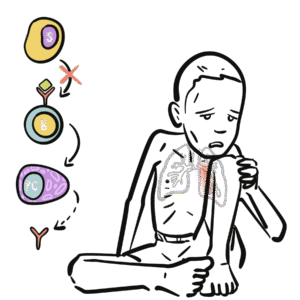
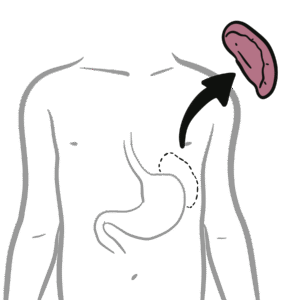
0:00 Now that we know a bit about antibodies, a bit about immunoglobulins, let's 0:10 look at 0:11 these different isotopes in a lot more detail. 0:15 So just recapping, there are five immunoglobulin isotopes based on the constant 0:21 region of the 0:22 antibody, these are IgM, IgD, IgG, IgE, and IgA. 0:34 Now we will first, and of course there are designated Greek symbols as well. 0:38 Here I'm drawing the bloodstream just to explain it better I guess. 0:44 Now we will first focus on IgG. 0:48 Now IgG has a molecular weight of 150 kilo-daltons. 0:55 We are also focusing on IgG first because it is the main immunoglobulins in the 1:00 blood, 1:01 lymph, cerebrospinal fluid and perineal fluid. 1:04 It actually forms 15% of total serum proteins, which is a lot. 1:12 And so they actually circulate in the blood as a monomer, as a single antibody. 1:17 And they are the most abundant. 1:19 And their half-life is about 23 days. 1:24 Now what's interesting about IgG, because it is abundant, it has many sub 1:28 classes, and 1:29 they differ in the length of the constant region of the heavy chain. 1:34 These subclasses, as I mentioned earlier, is IgG 1, 2, 3, and 4. 1:40 And deficiencies in any of these cause is a sort of indicator of specific 1:46 things, such 1:47 as a deficiency in IgG4 implies allergy, easily being allergic. 1:55 And to make this even more confusing, IgG2 can be further divided into IgG2A 2:00 and IgG2B. 2:01 Both have opposite effects. 2:02 IgG2A is for promoting inflammation, IgG2B is for promoting antibody production 2:10 . 2:11 So now, let us see what IgG does in the body, and how it helps the body fight 2:18 infection, 2:18 fight pathogens. 2:20 And we're focusing on IgG2 also and its functions, because some of the 2:25 functions of IgG are shared 2:27 by other antibody classes, such as IgM and IgD. 2:32 So the first thing we'll look at is, what IgG does in the body is perform obst 2:39 inization. 2:41 And this is essentially when it coats a pathogen, so that the body can easily 2:47 destroy it. 2:48 So for example, here we have a bacteria, and the bacteria contains antigens. 2:54 The IgG antibody can bind onto the antigen of the bacteria through the fab 3:00 portion. 3:01 And as it binds to the bacteria with the fab portion, the FC portion is 3:07 sticking out. 3:08 And with the FC portion sticking out, the FC portion can be easily detected by 3:13 phagocytes, 3:14 such as a macrophage, because a macrophage has receptors for the FC portion of 3:20 IgG. 3:21 And so when it binds, the bacteria, which is bound to the antibody, which is 3:27 bound to 3:27 the phagocyte, the phagocyte can easily engulf the bacteria, essentially. 3:34 The second thing IgG can perform is what's called agglutination. 3:39 And this is where it can essentially form precipitate. 3:43 It can form soluble antigens that allows it to be more easily phagocyzed. 3:51 So what I think this means is that when you have a lot of bacteria circulating 3:56 around, 3:56 for example, the IgG can basically bind onto these different bacteria and bind 4:01 onto each 4:01 other, forming sort of a clump, a cluster. 4:05 And so this allows the body to easily detect it and destroy all these bacteria 4:12 at once. 4:13 The most important thing I think to take in about IgG is that it actually 4:19 transfers to 4:20 the placenta, because it is the only immunoglobulin that actually passes 4:25 through the placenta, 4:27 excluding IgG2. 4:29 So what this means is that the mother, for example, has IgG antibodies for 4:35 specific things. 4:36 This immunity will pass on to the baby. 4:41 And this is facilitated by the IgG receptor on the placenta, which is expressed 4:49 about 4:49 three to four months into pregnancy. 4:52 So it is the only antibody that is transferred to the baby from the mother. 4:58 Another thing antibodies such as IgG can do is what's called antibody-dependent 5:03 cell-mediated 5:04 cytotoxicity. 5:06 And this is sort of similar to optimization in a way, in that if you have just 5:11 cells, 5:12 and then there is an abnormal cell amongst these cells, such as a tumor cell, 5:17 the fab 5:17 region can bind onto specific things that are expressed by this tumor cell. 5:24 And then it will obviously have the FC region sticking out. 5:27 This will allow cells called natural killer cells to bind on to the FC region 5:32 of the antibody, 5:34 and to realize that this is a tumor cell there, and so it will release cytotox 5:39 ins to kill it. 5:43 IgG also functions to neutralize toxins, and it can neutralize toxins such as 5:51 tetanus, 5:52 botulism, and snake and scorpion venom. 5:56 IgG acts by attaching to active sites of the toxins, basically inactivating it. 6:05 And because of this, in an event of an exposure, IgG is usually administered to 6:10 neutralize 6:11 the venom, the toxin. 6:14 And also, IgG works to neutralize viruses as well, and it binds to the viral 6:20 epitopes, 6:21 the antigens, and may prevent viral absorption and release. 6:29 Another function, lastly, that I haven't mentioned and that I cannot actually 6:33 fit in 6:33 this diagram, which is extremely important, is that IgG is one of the 6:38 antibodies that 6:39 can activate complement. 6:43 Complement is massive in that it stimulates an immune response. 6:50 And there's a video on complement. 6:51 I have a video if you want to watch it, but essentially IgG activates the 6:54 classical pathway 6:55 of complement. 6:57 So that was for IgG, the properties and functions of IgG. 7:02 However, some of the properties and functions of IgG are also shared with other 7:07 immunoglobulin 7:08 isotopes, such as IgM, and we'll look at IgM now. 7:14 So IgM has a molecular weight of 900 kilodaltons, which is massive. 7:20 It actually forms a pentameric structure in the bloodstream when it's once it's 7:24 secreted, 7:25 and it's held together by disulfide bonds, officially called the J-chain. 7:31 So here we have the pentameric structure of IgM held together by the J-chain. 7:38 Interestingly enough, IgM is the first antibody to be produced and expressed 7:42 following exposure 7:43 to an infectious agent. 7:45 Whereas IgG was the most abundant, IgM is the first to be produced. 7:51 And it has a relatively short half-life, about five days. 7:56 Because of IgM's pentameric structure, it makes it effective in activating 8:02 complement 8:02 and agglutination, activating complement as in activating the classical pathway 8:07 of complement. 8:09 And agglutination essentially, here we have a lot of bacteria, the IgM can form 8:14 precipitates, 8:15 basically forming clumps over together. 8:18 However, IgM has poor toxin and viral neutralization, and it does not cross the 8:25 placenta, because 8:27 remember IgG is the only antibody that crosses a placenta. 8:31 But even though IgG is the only antibody that crosses a placenta, IgM is 8:36 actually the first 8:38 to be made, and the only immunoglobut made by the fetus at five months. 8:44 IgM also has isohima gluten in activity, which I will not explain what it is, 8:49 you just 8:50 have to research that. 8:54 Now let's look at IgG. 8:56 IgG has a molecular weight of 180. 9:00 It is not a secreted immunoglobulin, but it is a membrane about immunoglobulin. 9:06 So for example, here we have B cells. 9:11 The B cells and plasma cells, they can express IgG on the membranes. 9:18 But also the B cell can express IgM. 9:22 Because IgG is membrane-bound immunoglobulin, we would expect to find low 9:27 concentrations 9:27 of it in the serum, in the bloodstream. 9:30 And also IgG can be co-expressed on B cells. 9:35 So the B cell, which has not become a plasma cell, can have both IgG and IgM. 9:42 And so IgG seems to be a marker for B cell maturity. 9:47 So here we have a B cell expressing both IgM and IgG. 9:52 It's not a plasma cell yet. 9:54 Whatever IgM is the first antibody to be expressed, IgG can be expressed. 10:02 And if the B cell is IgG, it means it is more mature. 10:06 However, the full function of IgG is still unknown. 10:11 Next is IgE, which has a molecular weight of about 200 kilodaltons. 10:15 It is secreted as a monomer, as a single antibody. 10:18 It has a half-life of about two days, but the half-life can increase if IgE is 10:25 bound 10:25 onto mast cells or basophils. 10:28 So for example, here we have IgE antibodies in the bloodstream, and here we 10:32 have a mast 10:33 cell. 10:34 IgE, the FC portion, can bind onto receptors on the mast cells. 10:39 And this will increase the half-life of IgE. 10:41 IgE, however, does not agglutinate and does not fix complement. 10:50 So as I mentioned, IgE has high affinity, well, it has high affinity for mast 10:55 cells and 10:55 basophils, which allows its half-life to be increased to about two weeks. 11:03 So here we have a mast cell, and the mast cell has special receptors called FC- 11:10 Epsilon-receptors, 11:12 which are for IgE antibodies. 11:16 And when it binds to these, the IgE can actually then bind onto antigens of 11:22 pathogens. 11:23 And when it does this, it will stimulate the mast cell to release its granules 11:28 containing 11:28 histamine. 11:30 Cytamine is an inflammatory mediator, which is important in the hypers 11:35 ensitivity reaction. 11:37 Interestingly enough, this mechanism is what we see in allergic reactions when 11:42 there is 11:43 histamine being released by mast cells. 11:47 The last antibody class I wanted to talk about is IgE, which has a molecular 11:51 weight of about 11:52 165 kilodaltons. 11:55 It can be secreted as a monomer, single antibody, or as a dimer, particularly 12:00 in the mucosal 12:01 system. 12:02 And therefore, IgA is important in the respiratory tract, the genital tract, 12:08 and the gut, the 12:10 mucosal system. 12:11 So here in the blood, we can have IgA monomer or IgA dimer. 12:17 If it's in a dimeric form, it is held together by what's called the J-chain. 12:21 And remember, the J-chain is what we saw at IgA dim. 12:27 It has a half-life of about 5.5 days, and it is the main immunoglobulin in the 12:33 mucus, 12:34 so in the respiratory tract, the gut, and the vaginal penis area. 12:39 Now there are two subclasses of IgA. 12:42 There's IgA1, which makes up 93% of all IgAs, and there's IgA2, which makes up 12:47 7% of all 12:47 IgAs. 12:48 And as I mentioned, it is the main immunoglobulin in the mucus, in the mucosal 12:53 system. 12:54 And so has the primary defense against mucosal infections, such as HIV and 12:59 helicobacter pylori 13:01 and strep pneumonia, which attacks the lungs. 13:05 IgA does not fix complement, activate complement, because we don't want an 13:11 inflammatory response 13:11 occurring in the mucosal tract. 13:15 It does, however, have agglutination and antiviral capacity, and it must have 13:18 antiviral capacity 13:19 because there are many viruses that enter the body through the mucosal system. 13:24 So that was for IgA. 13:26 I actually forgot to mention something about IgE, and that IgE has a function 13:32 in that it 13:32 has some form of function in protection against parasites, such as worms. 13:37 So that concludes the video on immunoglobulins. 13:39 As a summary, IgM is the first antibody to be expressed, produced. 13:45 IgD is a membrane-bound antibody. 13:48 IgG is the most abundant antibody in the blood, and does the most things in the 13:57 body, in terms 13:58 of protection. 14:00 IgE has, stimulates the hypersensitivity reaction, allergic reaction, and IgA 14:07 is the most abundant 14:09 amino globular in the mucosal system. 14:14 Thank you for watching. 14:15 I hope you enjoyed it.