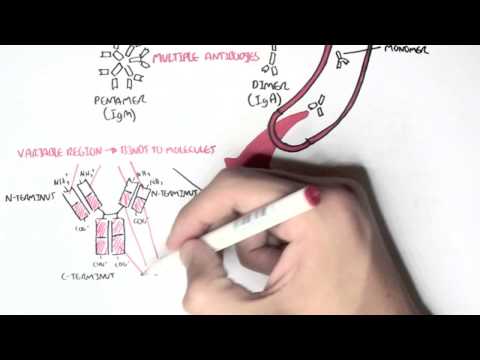
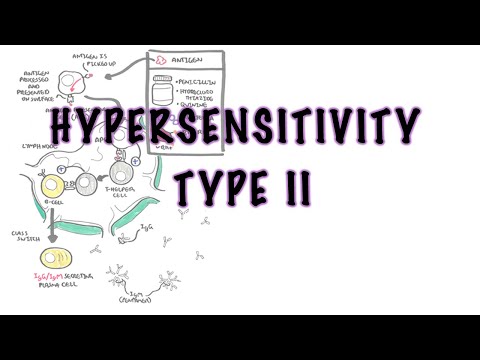
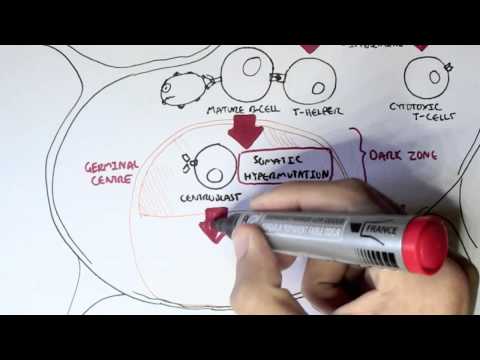
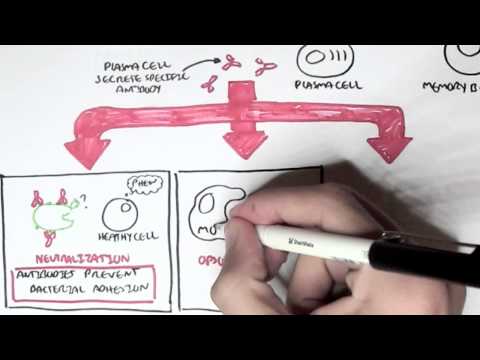
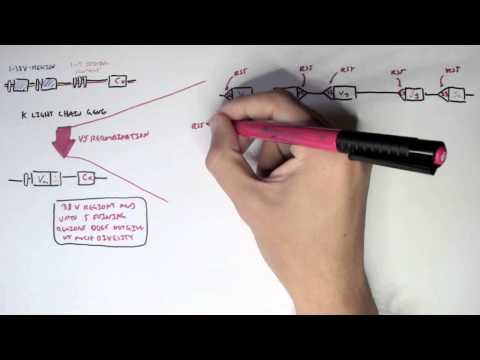
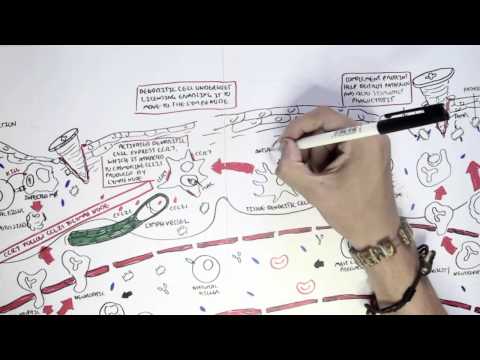
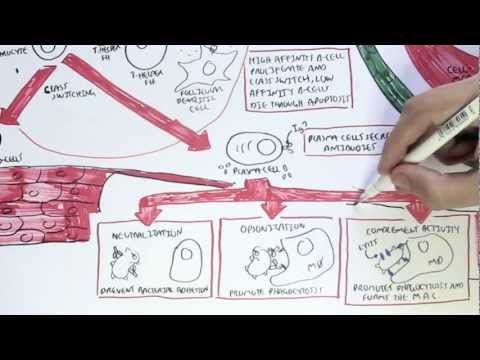
0:00 In this video we're going to talk about antibody-mediated transplant rejection. 0:12 The recipient or the host is the person who receives the donor organ. 0:17 The recipient can receive a renal transplant, a pancreatic transplant, and a 0:23 liver transplant. 0:24 The donor organ has its own unique antigen that is different to the recipients. 0:30 They can be labeled as foreign antigens. 0:34 When a recipient receives a donor organ, the recipient or the host's immune 0:40 system will 0:41 try and defend itself by mounting an immune response. 0:45 This immune response towards the donor organ is what leads to rejection and the 0:50 transplanted 0:51 organ dysfunction. 0:57 There are two main types of transplant rejection, T-cell mediated and antibody- 1:02 mediated rejection. 1:04 The T-cell mediated transplant rejection can be divided into acute or chronic 1:09 rejection. 1:10 Antibody-mediated transplant rejection is divided into hyperacute, acute, and 1:15 chronic. 1:16 In order to reduce and prevent an organ rejection, patients who receive an 1:21 organ transplant 1:23 take immunosuppressive agents to suppress their immune system. 1:28 In this video we will focus on antibody-mediated rejection and try to 1:33 understand the pathophysiology 1:35 and treatment. 1:40 Each and every individual has a unique tag or code. 1:44 This donor tissue or organ contains unique human leucocyte antigen, HLA, as 1:51 well as any 1:52 other antigen. 1:54 When it comes in contact with the host's immune system, the host's antigen 2:00 presenting 2:00 cells will recognize the tissue as foreign. 2:03 It engulfs the tissue and their antigen and presents the foreign antigen to the 2:10 host's CD4T 2:12 cells, also known as the T helper cells. 2:15 The antigen presenting cell activates the T helper cells, which in turn will 2:21 activate 2:21 B cells to become plasma cells. 2:24 Alternatively, the B cells and the T helper cells can co-stimulate, activating 2:33 each other. 2:34 It plasma cells will then produce antibodies towards the foreign HLA molecule 2:40 or other 2:41 foreign antigen parts. 2:44 The antibodies bind to the donor cells and trigger a number of downstream 2:49 events that 2:50 ultimately lead to the immune system attacking the donor tissue. 2:54 This is termed antibody-mediated rejection. 3:00 One of the first things that happen is that the antibodies can bind to foreign 3:04 HLA and 3:05 activate the complement system, a series of proteins that when activated 3:09 triggers cascade 3:10 of complement protein activation. 3:13 One of the activated proteins is C3A, which stimulates monocytes, macrophages 3:21 and neutrophils. 3:23 This is the same for C5A, which is a split product caused by C5-convertease. 3:32 C5B, the other product, help form the membrane attack complex, or MAC for short 3:38 , composed 3:39 of C5 to C9 complement proteins. 3:44 The membrane attack complex causes cell lysis and ultimately cell injury. 3:50 The injured donor cells will release chemicals that will activate surrounding 3:55 endothelial 3:56 cells to express adhesion molecules and release cytokines and chemokines to 4:00 help attract other 4:01 inflammatory immune cells to the area, such as neutrophils and macrophages. 4:11 These activated immune cells further release cytokines, which recruit other 4:14 immune cells, 4:15 including natural killer cells, which will attack the donor cells. 4:23 Endothelial cell injury adactivation leads to hemostasis, where you have plate 4:28 let activation 4:29 and thrombosis. 4:31 Thrombosis, if uncontrolled, may lead to ischemia of the tissue due to occl 4:36 usion in the blood 4:37 vessel. 4:40 You can see visually that antibody mediated rejection of a transplanted organ 4:46 is complicated. 4:47 The initial interaction between the host's antigen-presenting cell and the host 4:52 's T-helpercell 4:53 is the indirect pathway. 4:57 The direct pathway is when the donor's antigen-presenting cell, which is now 5:01 present in the recipient's 5:03 body, presents the foreign antigen directly to the host's T-cell. 5:18 Antibody mediated rejection can be divided into hyperacute, acute, and chronic. 5:23 So what are the differences? 5:26 Hyperacute rejection occurs due to preformed donor-specific antibodies present 5:31 in high 5:31 amounts before transplantation occurs. 5:35 Donor-specific antibodies are formed if a person has had a prior transplant, 5:41 blood transfusion, 5:42 or because of transmission from the mum. 5:46 Hyperacute rejection presents as graft rejection within minutes to hours after 5:51 transplantation. 5:53 Now it's rare thanks to better HLA matching and ABO compatibility. 6:04 Acute rejection usually occurs when the immunosuppressive agent someone is 6:09 taking is reduced. 6:10 The immunosuppressive agent is unable to withhold or stop the host's immune 6:16 system from 6:16 attacking it, and as a result, acute rejection occurs. 6:21 Immunosuppression can occur at any time during the transplant period and can be 6:26 days to weeks. 6:28 To prevent acute rejection, careful immunosuppression tapering and dose 6:36 adjustment is required. 6:39 Chronic rejection is usually due to non-adherence to immunosuppression, or 6:43 difficulty in increasing 6:44 the immunosuppressive agent for whatever reason. 6:47 As a result, the host slowly develops low titers of donor-specific antibodies 6:53 over time, which 6:54 causes rejection and failure of the solid organ. 7:02 An important investigation to distinguish antibody-mediated rejection to cell- 7:08 mediated rejection is by 7:09 measuring the complement protein which works alongside the antibodies, 7:15 specifically complement 7:16 protein 4D, which is a marker of antibody-mediated rejection. 7:22 Once antibody-mediated rejection is established, what are the treatment options 7:26 available? 7:27 Well the treatment is immunosuppression because you want to dampen the immune 7:32 system. 7:33 The choice of immunosuppressive agents will depend on the organ affected and 7:37 also whether 7:38 there is an acute or chronic rejection. 7:41 In general, multiple immunosuppressive agents are used, and they can include 7:45 the following. 7:47 Firstly, increasing glucocorticoids, which work by inhibiting the immune cell 7:53 function 7:54 on multiple levels. 7:56 But these come with many side effects, which I will not discuss, but include 8:00 diabetes and 8:01 osteoporosis. 8:03 The phosphoresis removes plasma from the blood, which contains the donor- 8:10 specific antibodies. 8:12 The removed plasma is then replaced with a substitute. 8:16 Intravenous immunoglobulins is essentially pooled antibodies given to a patient 8:22 . 8:22 The ultimate goal of this therapy, or the mechanism is broad, but it is to 8:27 normalize 8:28 a immune system, essentially. 8:32 The side effects of intravenous immunoglobulins include arthralgia, myelges, 8:39 and hypertension. 8:41 Equalizumab is a monoclonal antibody directed against the C5 fragment of the 8:46 complement 8:47 cascade, and inhibits the generation of the membrane attack complex we have 8:52 learned. 8:52 The side effect is that, mainly, it increases the risk of Niceria meningitidis 9:00 infections. 9:01 Let's look at a zoomed view of the interaction between the T-helper cell and 9:05 the B-cell. 9:06 The T-helper cells activate the B-cells to mature to become plasma cells. 9:11 It does this by binding to receptors and releasing cytokines. 9:17 Baletecept specifically binds to B7 receptors, specifically the CD86 subtypes, 9:24 that are 9:25 found on the antigen-presenting cells, such as the B-cells. 9:29 Baletecept prevents co-stimulation between T and B-cells. 9:35 The side effect of Baletecept is that it increases risk of cytomegalovirus, 9:40 Epstein-Barr virus, 9:42 and post-transplant lymphoprolative diseases. 9:48 Alum2zumab is a humanized monoclonal antibody against CD52 on lymphocytes. 9:55 This then mediates lymphocyte destruction through a number of mechanisms. 10:00 The side effect of Alum2zumab is the O2 immune phenomenon. 10:06 It increases the risk of things such as ITP, Graves' disease, and anti-GBM. 10:17 Rituxumab is an anti-CD20 monoclonal antibody. 10:21 It depletes B lymphocytes that expresses CD20 on their surface. 10:25 The side effects are many, but the main one is that it increases the risk of 10:30 progressive 10:30 multifocal leukoenkylopathy. 10:36 Tosa-Lizumab is an antibody against the interleukin-6 receptor. 10:41 Interleukin-6 is an important inflammatory cytokine. 10:46 Inhibiting interleukin-6 receptor means interleukin-6 cannot trigger 10:50 inflammation. 10:51 The side effects of Tosa-Lizumab include hypertension and hyperlipidemia. 10:57 Cyclophosphamide is an alkylating agent and inhibits the cell cycle. 11:00 It reduces B and T-cell development. 11:05 Side effects include hemorrhagic cystitis and gonatal failure. 11:13 In summary, the immunosuppressive agents use to treat antibody-mediated 11:18 rejection or any 11:19 rejection for that matter comes with many side effects. 11:23 This includes one, it increases the risk of infection because you're 11:27 suppressing the immune 11:28 system. 11:29 It increases the risk of reactivation of basically dormant infection or later 11:34 infections. 11:35 It increases the risk of malignancy. 11:37 There's obviously a risk for transfusion reactions as well as allergy. 11:44 In summary, chronic rejection develops slowly with antibodies produced in low 11:50 titers. 11:51 Chronic rejection is likely due to poor compliance or issues with taking immun 11:54 osuppressive agents. 11:56 Along the way, the host may develop acute rejections and this can be due to a 12:00 reduction 12:01 in their current immunosuppressive dose. 12:04 In this scenario, the logical thing to do is to increase their immunosupp 12:08 ressive agent 12:09 again. 12:10 It's about finding the right dose of immunosuppression, making sure the 12:13 transplant organ is functioning 12:15 well and managing the complication of the immunosuppressive agents. 12:20 Thank you for watching, I hope you enjoyed this video. 12:34 (upbeat music)