Eosinophilic Granulomatosis with Polyangiitis
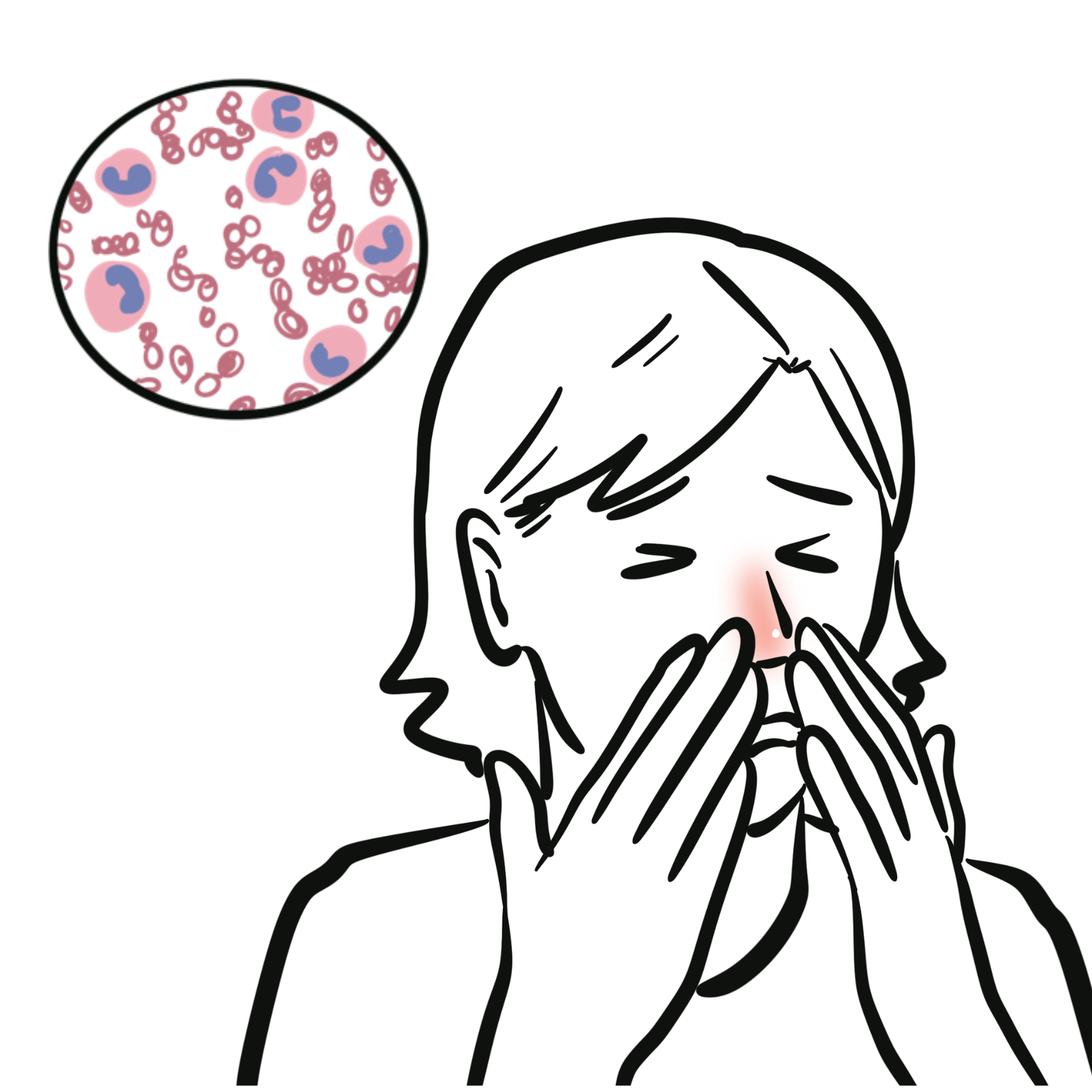

EGPA (formerly Churg-Strauss syndrome) is a rare, small-to-medium vessel necrotizing vasculitis characterized by asthma, eosinophilia, and extravascular granulomas. It commonly affects the lungs, skin, peripheral nerves, and heart. Annual incidence: ~1–3 per million; peak onset in 40s–60s; no significant gender bias. Strongly associated with MPO-ANCA positivity in ~40–60% of patients; others are ANCA-negative.
Eosinophilia: abnormally high number of eosinophils in the blood or tissues, which is a hallmark feature of EGPA and often appears before vasculitic symptoms.
Granuloma: localized collection of inflammatory cells, such as macrophages and giant cells, and in EGPA these extravascular granulomas contribute to tissue and organ damage.
ANCA (Anti-Neutrophil Cytoplasmic Antibodies): autoantibodies against proteins in neutrophils, found in around 40% of EGPA patients, and are linked to clinical features such as glomerulonephritis and neuropathy.
Eosinophil Function
| Key Cytokines in Eosinophil Production and Activation | |
| Cytokine | Function |
| IL-5 | Most important; stimulates eosinophil growth, differentiation, activation, and survival in tissues |
| IL-3 | Promotes early eosinophil progenitor development from bone marrow |
| GM-CSF | Enhances eosinophil activation and prolongs survival |
| IL-4 / IL-13 | Promote Th2 differentiation, leading to more IL-5 production |
| Eotaxins (CCL11, CCL24) | Chemokines that recruit eosinophils to sites of inflammation (especially in lungs and GI tract) |
IL-5 is the central cytokine in eosinophilic disorders. Targeting IL-5 (e.g., with mepolizumab) is effective in EGPA, severe eosinophilic asthma, and HES.
Aetiology
Risk Factors
If a patient with eosinophilic asthma develops neuropathy, purpura, or systemic symptoms, always consider EGPA.
Cardiac involvement is the most important prognostic factor.
Unlike GPA/MPA, mononeuritis multiplex and cardiac involvement is common in EGPA.
Classification Criteria (2022 ACR/EULAR)
| Entry Requirement: A diagnosis of small- or medium-vessel vasculitis has been made (biopsy), and other mimicking conditions have been excluded. | |||
| Variables | GPA | MPA | EGPA |
| Clinical criteria | |||
| Nasal passage involvement | +3 | −3 | — |
| Cartilaginous involvement | +2 | — | — |
| Conductive or sensorineural hearing loss | +1 | — | — |
| Obstructive airway disease | — | — | +3 |
| Nasal polyp | — | — | +3 |
| Mononeuritis multiplex | — | — | +1 |
| Laboratory criteria | |||
| PR3-ANCA (or C-ANCA) positivity | +5 | −1 | −3 |
| MPO-ANCA (or P-ANCA) positivity | −1 | +6 | — |
| Serum eosinophil ≥1000/µL | −4 | −4 | +5 |
| Hematuria | — | — | −1 |
| Histological criteria | |||
| Granuloma, granulomatous inflammation, or giant cells | +2 | — | — |
| Pauci-immune glomerulonephritis | +1 | +3 | — |
| Extravascular eosinophilic-predominant inflammation | — | — | +2 |
| Radiological criteria | |||
| Pulmonary nodules, mass, or cavitation on chest imaging | +2 | — | — |
| Fibrosis or ILD on chest imaging | — | +3 | — |
| Nasal/paranasal sinusitis or mastoiditis on imaging | +1 | — | +1 |
| Total Score Cut-off for Classification | ≥5 | ≥5 | ≥6 |
Investigations
Differential Diagnoses
| Diagnosis | Key Differences |
| Eosinophilic asthma | Chronic airway inflammation with mild to moderate eosinophilia. Only lung involvement, no systemic vasculitis. |
| Hypereosinophilic syndrome | Persistent eosinophilia without vasculitis or parasitic infection |
| Allergic bronchopulmonary aspergillosis | Asthma + eosinophilia, but lacks systemic vasculitis |
| Phase | Treatment |
| Mild (non-severe) | Glucocorticoids taper ± Mepolizumab |
| Severe (organ/life-threatening) | High-dose glucocorticoids + cyclophosphamide or rituximab |
| Maintenance | Glucocorticoid taper + Mepolizumab, azathioprine, methotrexate, or rituximab |
Supportive
Complications
Prognosis

Please confirm you want to block this member.
You will no longer be able to:
Please allow a few minutes for this process to complete.
Discussion