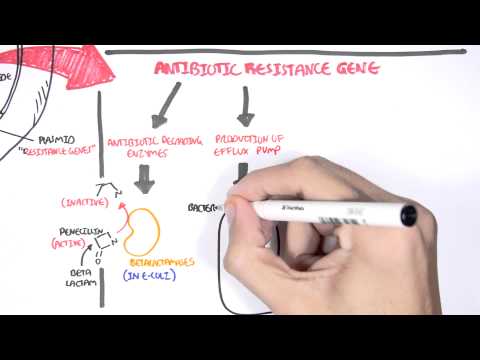
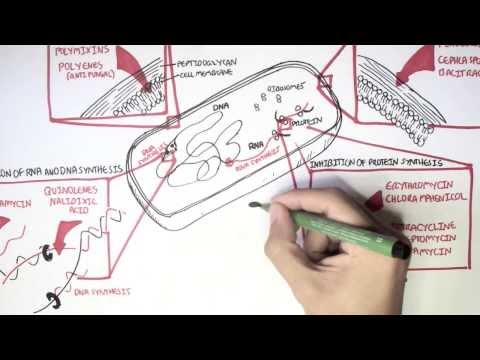
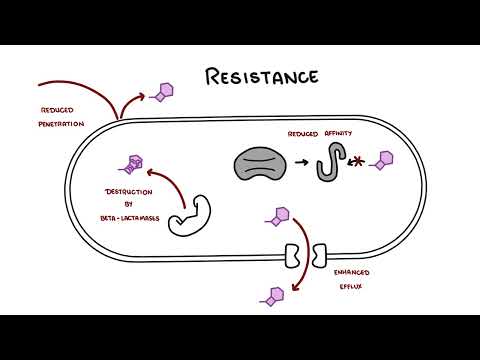
0:00 Hello, in this video we're going to talk about penicillins, the antibiotic, 0:08 which was first 0:09 discovered by a guy called Alexander Fleming in 1928, but it did not become 0:14 widely available 0:14 until the 1940s. 0:16 The penicillins are closely related compounds comprising a beta-lactam ring, a 0:22 five-membered 0:23 theosolodyne ring, and a side chain. The ring structures are essential for 0:29 antibacterial 0:30 activity and the side chain determines the spectrum and pharmacological 0:35 properties. Most 0:36 penicillins in current use are semi-synthetic derivatives of six amino penic 0:42 ilic acid. 0:43 Penicillins inhibit bacterial cell wall synthesis and thus are bactericidal, 0:49 meaning that they 0:50 kill bacteria. 0:53 So, what is the classification of penicillins? Well, first of all, remember 1:00 that penicillins 1:01 are beta-lactam antibiotics because they're the beta-lactam ring, but they're 1:05 also other 1:05 antibiotics that also have a beta-lactam ring in their molecular structure. 1:10 These are your 1:11 kephylosporins or cephalosporins, some people say, monobactams and carbapenins. 1:19 Specifically 1:20 talking about penicillins, penicillins itself can be classified into six big 1:25 groups. 1:26 Group one are your benzyl penicillins and it's a long-acting parenteral form. 1:31 Group 1:31 six are your orally absorbed penicillins, such as phenoxy methyl penicillin. 1:36 Group three 1:37 are your anti-staphylococcal penicillins, such as your flucloxacillins. Group 1:42 four are 1:43 your extended spectrum penicillin, such as amoxicillin. Group five have anti- 1:49 pseudomonal 1:50 properties, your anti-pseudomonal penicillin, such as peppericillin. Group six, 1:55 beta-lactamase 1:57 resistant penicillins. So, how do penicillins work? Well, penicillins inhibit 2:07 cell wall synthesis 2:09 of bacteria. So, bacterial cell wall synthesis. And they do this by binding to 2:14 what's called 2:15 penicillin binding proteins or PBPs. Now, PBPs are really important in cell 2:25 wall synthesis. 2:27 And so, when you have a penicillin, as shown in this diagram, penicillin 2:31 essentially latches 2:32 on to the PBPs, the penicillin binding proteins, and inhibit what's called the 2:38 transpeptidation 2:39 of peptidoglycans. And so, therefore, inhibit cell wall synthesis causing an 2:46 unstable cell 2:47 wall leading to bacterial death. Now, penicillins and other antibiotics are 2:58 very glamorous because 2:59 they really inhibit bacteria. They kill bacteria when people are sick. However, 3:04 bacteria are 3:05 also very glamorous and very smart because they have certain enzymes and 3:10 properties that 3:10 actually allow them to resist certain antibiotics, such as penicillins. And 3:15 bacteria may become 3:17 resistant to penicillins through a number of mechanisms. First of all, through 3:23 the destruction 3:24 of the actual antibiotics by enzymes called beta-lactamases. This is the most 3:29 commonest 3:30 way bacteria inhibit essentially penicillin function. Other forms of resistance 3:38 to antibiotics, 3:39 such as penicillin, includes the failure of penicillin to actually penetrate 3:44 the outer 3:44 membrane of gram-negative bacteria. Bacteria can have pumps that essentially 3:51 push out the 3:53 antibiotic outside the bacteria, specifically in gram-negative bacteria. And 4:02 also, there's 4:03 low affinity binding of the penicillins to target penicillin binding proteins. 4:10 Some bacteria 4:10 may display more than one resistant mechanism, such as in MRSA. 4:17 Let's talk a bit more about beta-lactamase. Now, as mentioned, beta-lactamase 4:24 is our 4:24 enzymes that bind covalently to the beta-lactam ring of antibiotics and they 4:30 hydrolyze it 4:31 and make the antibiotics ineffective, and so the bacteria survives. And this is 4:37 one way 4:38 bacteria develop resistance because without an effective beta-lactam 4:41 antibiotics, such 4:42 as penicillin, the bacteria survive. Now, there's this thing called beta-lactam 4:48 ase 4:48 inhibitors that actually stop beta-lactamase and therefore restore the antib 4:53 acterial properties 4:55 of the beta-lactam antibiotics. There are three beta-lactamase inhibitors that 5:02 are in 5:02 clinical use, big ones, such as clavolinic acid, sulfactam, and tazobactam. All 5:11 are 5:11 only available in combination with a beta-lactam antibiotic. So these include 5:18 coamoxaclav, 5:19 peppericillin with tazobactam, also known as tazosin. 5:25 So where are penicillins used? Well, they're using a lot of infections. For 5:35 example, 5:36 benzylpenicillin is used in infections due to group A and group B, streptococci 5:40 , 5:40 meningitis due to strep pneumoniae, and neccesiria meningitis, streptococcal 5:46 and enterococcal endocarditis, neurosyphilis. Amino penicillins are used in 5:50 respiratory 5:51 tract infections, endocarditis, meningitis, extended spectrum and anti-sooder 5:58 modal penicillins 5:58 are used in infections due to resistant gram negative bacteria. And then the 6:04 phenoxy methyl 6:04 penicillins are also used prophylactically to prevent recurrent rheumatic fe 6:08 vers, or for 6:10 example, used to help patients who don't have a spleen because they're an 6:15 increased risk of 6:16 encapsulated infections. So let's talk a bit more about the pharmacology of pen 6:26 icillins. 6:26 Penicillins differ really markedly across a spectrum in their oral absorption. 6:31 So for example, 6:32 phenoxy methyl penicillins is 60% orally absorbed, whereas anti-soodermodal pen 6:37 icillins 6:38 zero, and are usually given parentially IV. They vary in their degree of 6:44 protein binding, 6:46 and metabolism is minimal. Penicillins are rapidly excreted via renal tubular 6:53 cells, 6:53 and excretion may be blocked by probenicid, a medication used in gout. Dose 6:58 modification is 6:59 important in renal failure. Finally, adverse effects and toxicity. Now, 7:11 allergic reactions 7:12 are common, and this includes a screen skin rash, serum sickness, delayed 7:17 hypersensitivity, 7:17 anaphylactic reactions are very rare. People often have GI upset, such as 7:23 diarrhea, 7:24 intericalitis can be seen, especially with ampicillin. Hematological 7:29 abnormalities, 7:30 such as hemolytic nemia, cytopenia, such as neutropenia and thrombocytopenia 7:35 can also be seen 7:36 in up to 4%. Importantly, penicillins can also cause an elevation in transamin 7:41 ases, 7:42 usually flucloxacillin, as well as mild electrolyte abnormalities, such as 7:46 hypernaturmia. 7:48 Renal adverse complications include interstitial nephritis and hemorrhagic cyst 7:53 itis. C&S complications, 7:56 so central nervous system complications include encephalopathy and even 8:00 seizures, 8:00 which are extremely rare, but may occur, especially if someone has renal 8:05 failure or are on a prolonged 8:07 dose of penicillin. So, in summary, penicillin is a medication used to manage 8:15 and treat a wide 8:17 range of infections. It is a beta-lactam antibiotic, and there are many groups 8:24 or classes of penicillins, 8:26 but they essentially work by binding specifically to penicillin binding 8:30 proteins on a cell wall of 8:32 bacteria, and thus inhibiting the bacterial cell wall synthesis, causing it to 8:38 die. Thank you for 8:40 watching. 8:47 You