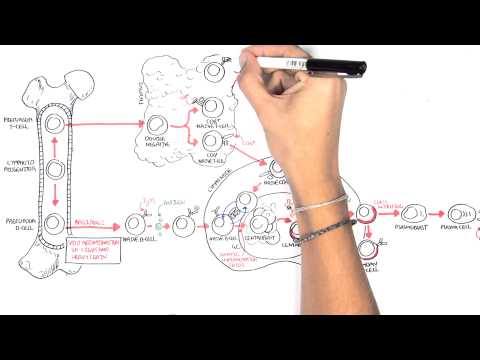
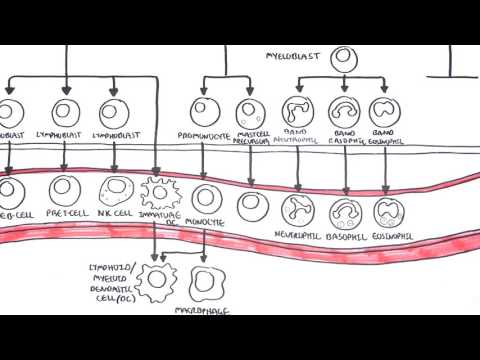
0:00 Hello, in this video, we're going to talk about hamato poasis. 0:10 Hamato poasis, as the name suggests, hamato refers to the blood and poasis is 0:16 essentially 0:17 I guess formation of. 0:19 And hamato poasis refers to the commitment and differentiation process of a 0:26 stem cell 0:27 to the different types of cells we found in the blood. 0:31 For example, our red blood cells. 0:34 Now, hamato poasis, which is the making of these different cells, they occur 0:41 mainly 0:41 in the bone marrow. 0:44 And this is particularly in adults. 0:47 This is referred to as medullary, hamato poasis. 0:51 But hamato poasis can also occur in other tissues such as the liver, thymus, 0:55 and spleen. 0:56 And this is called extra, medullary, hamato poasis. 1:00 And we'll learn about that in this video. 1:03 So let's begin by zooming into the bone marrow. 1:06 And in the bone marrow, we can find hamato poetics stem cells. 1:10 Hamato poetics stem cells can first of all differentiate into either what's 1:15 called a 1:15 common lymphoid progenitor cell or a common myeloid progenitor cell. 1:21 The common lymphoid progenitor cell then further differentiates into different 1:26 lymphoblasts 1:27 with different genetic profiles. 1:30 And this will allow them to form and mature into different types of lymphocytes 1:36 . 1:36 So here I'm just drawing the bloodstream because as cells exit the bone marrow, 1:41 they 1:41 will enter the blood. 1:43 So these different lymphoblasts with the different genetic profiles will become 1:51 either naive 1:51 B cells, pre-T cells, or natural killer cells. 1:57 Now also common lymphoid progenitor cells can differentiate into certain types 2:02 of dendritic 2:03 cells. 2:04 The dendritic cells, when it's formed, will move into tissues and becomes what 2:09 's known 2:10 as a lymphoid dendritic cells. 2:13 Dendritic cells are peripheral antigen presenting cells that are actually very 2:18 important in 2:19 the connection between the innate immune system and the adaptive immune system. 2:27 So that was the lymphoid progenitor cell lineage. 2:32 Now let's look at the common myeloid progenitor cell lineage. 2:36 And it's important to know that the outcome of these lineage are cells that can 2:44 circulate 2:44 in the blood, so hence hematopoietic. 2:48 So again, from the hematopoietic stem cell, it can become a common myeloid prog 2:54 enitor cell. 2:57 And there are different maps of hematopoiesis that depict common myeloid progen 3:03 itor cell 3:03 differentiation. 3:05 But how I'm going to show you is what I think is the easiest. 3:09 Anyway, the common myeloid progenitor cell can become myeloblasts and 3:15 eventually become 3:17 granulocytes. 3:18 These granulocytes are cells containing granules. 3:22 And they can either be band neutrophils, band basophils, and band isinophils. 3:31 So neutrophils, basophils, isinophils. 3:34 And these granulocytes are band cells because they are immature. 3:40 But once they enter the circulation, they mature and become what we know as 3:45 neutrophils, 3:46 basophils, and isinophils respectively. 3:50 Neutrophils are important in the acute response to infection and inflammation. 3:54 Basophils are important in allergy and parasitic infections. 3:59 Like isinophils are also important in allergies and sensitivity. 4:06 The common myeloid progenitor cells can differentiate and become promonocytes 4:12 and mast cell precursors. 4:14 The promonocytes then mature and become what we know as monocytes. 4:21 Monocytes are essentially circulating macrophages. 4:25 And so once they move into a tissue, such as the skin, they become tissue mac 4:32 rophages. 4:33 And macrophages are antigen presenting cells, which again are important in the 4:38 connection 4:38 between the innate immune system and the adaptive immune system. 4:45 The monocytes or promonocytes can also become dendritic cells. 4:52 And if they become dendritic cells in the tissue, they are referred to as the 4:57 myeloid 4:57 dendritic cells. 5:00 So again, we have two important antigen presenting cells, the macrophage and 5:06 the dendritic cell. 5:08 The muscle precursor can become mature muscles once they enter circulation and 5:15 move into 5:16 tissues. 5:18 Smells are very important cells in allergy, the inflammatory response and 5:23 hypersensitivity. 5:25 Finally, the common myeloid progenitor cell can differentiate and become mega k 5:32 aryocytes 5:32 with stimulation of thrombopoietin, a hormone produced by the liver and the 5:40 kidneys. 5:41 Or the myeloid progenitor cell can become erythroblasts through stimulation of 5:46 erythropoietin 5:48 a hormone released by the kidneys. 5:52 Let's look at mega karyocytes first. 5:55 Mega karyocytes are normally present in the bone marrow, not in the circulating 6:00 blood. 6:01 But these guys give rise to the platelets we find in the blood. 6:06 Mega karyocytes rupture releasing platelets into circulation and platelets are 6:11 very important 6:12 in clotting. 6:15 Erythroblasts are still nucleated red blood cells, meaning that they have a 6:23 nucleus. 6:23 But once these erythroblasts enter circulation and mature, they become erythro 6:29 cytes, which 6:31 are a nucleated. 6:33 Erythrocytes are our red blood cells. 6:38 In adults, healthy adults, hematopoasis occurs in the bone marrow, particularly 6:45 in the pelvis 6:45 – the vertebrae – and sternum. 6:48 However, hematopoasis can occur in other organs. 6:53 And this is called extra-modolary hematopoasis. 6:58 And let us look at a graph with X being the timeline. 7:02 Here is birth and down the X-axis to 70 years old. 7:07 And on the Y-axis is hematopoasis, where the blood cells, the different blood 7:12 cells 7:12 are made. 7:13 So before birth, so when you are a fetus in the uterus, hematopoasis occurs 7:21 predominantly 7:22 in the liver and the spleen. 7:24 And this is the fetus we're talking about. 7:25 So in the fetus, hematopoasis occurs in the liver and spleen. 7:29 But then it drops off by birth. 7:33 And this is because slowly the bone marrow will take over the role. 7:40 And by adulthood, the bone marrow has the main role in hematopoasis, 7:45 particularly the 7:46 vertebrae bone marrow and the pelvis and sternum. 7:50 Hematopoasis occurring in the bone marrow is termed modolary hematopoasis. 7:57 It's interesting to note that hematopoasis also occurs in the lymph nodes 8:01 during adulthood, 8:02 but at a much lower concentration and usually occur more often during periods 8:10 of infections. 8:12 So what is very interesting is that the liver and spleen, as one might suspect, 8:17 has no to 8:19 minimal role in hematopoasis after birth. 8:22 However, they actually do have a role, particularly during periods of 8:28 infections or during pathological 8:31 changes, such as in certain diseases. 8:37 When other organs perform hematopoasis asides the bone marrow, such as if it 8:42 happens in 8:43 the lymph nodes spleen or liver, this is called extramadolary hematopoasis. 8:52 When you think about it, a child with an infection, the liver or spleen can 8:57 sometimes 8:57 enlarge, and this is because the spleen or the liver is working harder to make 9:02 more blood 9:02 cells, but this is usually self-limiting and goes away. 9:11 Now the myeloproliferative disorders comprise several clonal hematological 9:17 diseases that 9:19 are thought to arise from a transformation in the hematopoietic stem cell. 9:25 The main clinical feature of these diseases is the overproduction of mature 9:30 functional 9:30 blood cells and a long clinical course. 9:35 There are many types of myeloproliferative disorders. 9:38 The main classic myeloproliferative disorders are three. 9:43 These three are polycythemia rubrivera, essential thrombocythemia, and primary 9:51 myelofibrosis. 9:53 Let's look briefly at what happens in each of these three. 10:00 If there's a mutation or abnormality somewhere along the pathway from the myel 10:04 oid progenitor 10:05 cell to erythropoasis, so the formation of red blood cells, this can lead to 10:11 overproduction 10:13 of erythrocytes. 10:14 This leads to the condition polycythemia rubrivera, which is characterized by 10:19 increased number 10:20 of circulatory red blood cells, circulatory erythrocytes. 10:26 Similarly, if there is a mutation along the megacaryocyte lineage, this can 10:33 lead to proliferation 10:35 of megacaryocytes and therefore overproduction of thrombocytes, the platelets, 10:41 and this will 10:42 lead to a condition called essential thrombocythemia. 10:47 Another thrombocythemia is characterized by a lot of circulatory platelets. 10:52 Finally, mutations occurring down certain parts, other myeloid progenitor cell 10:59 pathways, 11:00 can lead to myelofibrosis. 11:02 And myelofibrosis, as the name suggests, is fibrosis due to the myeloid cells. 11:09 Primary myelofibrosis is characterized actually by a megacaryocyte hyperplasia 11:15 and fibrosis 11:16 due to the increase in fibroblasts in the bone marrow, which because you have a 11:21 lot of fibroblasts, 11:22 they will deposit heaps of collagen and this in turn will lead to bone marrow 11:29 failure, 11:30 so the failure of the bone marrow to make more cells. 11:34 So I've talked about mutations along the myeloid progenitor cell pathway and 11:40 how it leads to 11:41 these three conditions that make up the myeloproliferative disorders. 11:47 Well, there are actually mutations that are shared amongst the myeloprolifer 11:52 ative disorders 11:53 and this mutation is the JAC2 signaling pathway mutation. 11:59 So because 90% of polycythemia ruevara have a JAC2 mutation and more than 50% 12:08 of essential 12:09 thrombocythemia and primary myelofibrosis also have a mutation in the JAC2 gene 12:16 . 12:16 The JAC2 gene actually is a gene that provides instructions for making a 12:22 protein that promotes 12:23 the growth and division of cells.