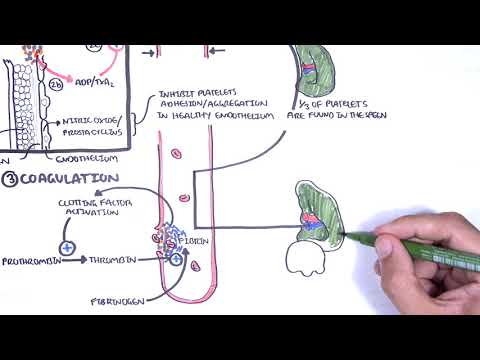
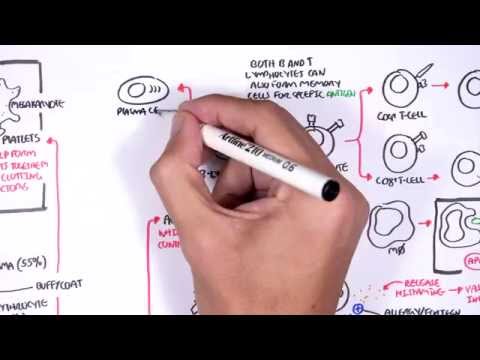
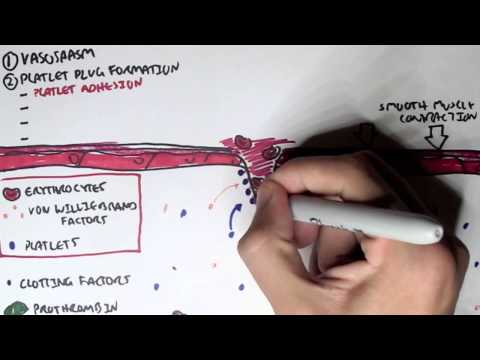
0:00 Hemostasis is divided into primary hemostasis, which involves platelet plug 0:10 formation and 0:12 secondary hemostasis, which is the coagulation cascade. 0:17 The classic coagulation cascade is divided into the extrinsic pathway and the 0:24 intrinsic 0:25 pathways, both of which lead to what is called the common pathway. 0:31 The hallmark of both the extrinsic and intrinsic pathways of coagulation is 0:35 this sequential 0:37 activation of a series of clotting factors. 0:41 Clotting factors are shown as Roman numerals, and numerals followed by the 0:45 alphabet represents 0:46 an active clotting factor. 0:48 For example, factor 10 becomes factor 10a, 10a being the active factor. 0:56 So let's focus on the extrinsic pathway. 0:59 Following platelet plug formation, tissue injury causes expression of tissue 1:05 factor, 1:05 which together with factor 7a will activate many molecules of factor 10 to 1:11 become factor 1:13 10a. 1:16 The intrinsic pathway is initiated by the exposure of blood to a negatively 1:20 charged surface, 1:22 which activates factor 12. 1:25 This in turn activates factor 11, 9, and then 8. 1:34 Factor, activated factor 8 will activate factor 10. 1:42 The intrinsic and extrinsic pathways will activate factor 10. 1:48 Factor 10a and factor 5a make up the beginning of the common pathway. 1:55 Factor 10a and 5a as the cofactor activates prothrombin to become thrombin, 2:02 which is our 2:02 factor 2a, the active form. 2:07 Fibrin, which are the factor 2a molecules, then convert fibrinogen to fibrin. 2:13 Fibrin is factor 1a. 2:17 Local generation of fibrin in turn enmeshes and reinforces the platelet plug, 2:22 which was 2:22 formed from the primary hemostasis. 2:26 Bleeding is successfully stopped. 2:29 It is important to know that calcium is involved in the clotting cascade in a 2:33 few steps. 2:35 This is a classical view of the clotting cascade based on the extrinsic and 2:41 intrinsic pathways. 2:42 The classical view is very useful in interpreting coagulation tests and 2:48 identifying bleeding 2:49 disorders based on the coagulation factors. 2:52 However, physiologically, the coagulation cascade is much more confusing. 2:57 There is a cell-based model for coagulation, which involves three main steps. 3:04 The first is initiation. 3:06 It is now believed the generation or exposure of tissue factor from the extrins 3:11 ic pathway 3:12 at the wound site and its interaction with factor 7a is the primary 3:18 physiological event 3:20 in initiating clotting leading to activation of thrombin. 3:25 The small initial amount of thrombin generated then activates factor 5, 11 and 3:31 8 in a feedback 3:32 manner leading to amplification of thrombin generation. 3:37 Interestingly, and on a side note, factor 8 is carried usually by Von Wilberin 3:42 factor 3:43 because it increases the factor's half-life. 3:46 Factor 2a release is factor 8 from Von Wilberin factor. 3:54 In summary, step 2 is amplification with a small amount of thrombin generated 3:58 from the 3:58 extrinsic pathway activates factor 5, 8 and 11. 4:03 This in turn activates the platelets. 4:07 Activated platelets and the activated factors earlier then leads to the 4:13 propagation step. 4:14 Activated factors here form the intrinsic tenase resulting in factor 10a 4:21 activation. 4:22 The activated factor 10 binds with factor 5a to form the prothrombinase complex 4:31 . 4:31 Prothrombinase complex converts rapidly prothrombin into thrombin directly onto 4:37 platelets 4:38 and subsequently forms fibrin. 4:43 So in summary, that was the cell-based model, initiation, amplification and 4:48 propagation 4:49 and ultimately prothrombinase complex which forms a lot of thrombin and 4:53 subsequently 4:53 a fibrin mesh stopping bleeding. 5:01 To restore vessel patency following hemostasis, the clot must be organized and 5:06 removed. 5:06 This is called fibrinolysis. 5:09 Fibrin gets broken down to fibrin degradation products including d-dimers by 5:14 the enzyme 5:14 plasmid. 5:17 Thrombin is derived from its precursor plasmidogen which is activated by plasm 5:21 idogen activator 5:22 TPA. 5:25 Interestingly, thrombin activates a molecule called thrombin activatable fibrin 5:31 olysis inhibitor 5:32 and as the name suggests, this inhibitor basically slows down fibrinolysis so 5:38 you want 5:39 to slow down the breakdown of the fibrin. 5:43 Now that we know the classic model of the coagulation cascade which involves 5:48 the intrinsic 5:49 pathway and the extrinsic pathway and then we learned about the cell-based 5:53 model which 5:54 includes initiation, amplification priming and propagation, it is important to 6:00 learn 6:00 about the control mechanisms and the termination of the clot or coagulation. 6:07 The coagulation cascade involves interaction of the activated platelets and the 6:10 clowing 6:11 factors with its propagation of thrombin and fibrin formation is good to stop 6:16 bleeding 6:16 and commence healing. 6:18 However, you can imagine if the coagulation cascade is continuous without a 6:24 break, a thrombus 6:25 can form leading to clots in the veins, the arteries causing organ damage. 6:31 The body thus has a few ways to stop and control clotting. 6:35 This is the anti-thrombotic pathway. 6:42 The first anti-thrombotic pathway to remember is protein C and protein S. As 6:50 clot formation 6:51 progresses, thrombin, which is factor 2A, binds to thrombo-modulin, an integral 6:57 membrane 6:57 protein on the endothelial cell surface. 7:02 The thrombin-thrombo-modulin complex activates protein C, which together with 7:07 protein S as 7:08 a cofactor inhibits factor 5A and factor 8A, activity, halting coagulation. 7:17 Another anti-thrombotic pathway is through tissue factor pathway inhibitor or T 7:21 FPI, which 7:22 circulates in plasma but at very low concentrations. 7:25 It works in the extrinsic pathway and inhibits factor 10 activation in two main 7:31 ways. 7:31 It directly inhibits factor 10A and it inhibits tissue factor and faffin A. 7:39 The other anti-thrombotic pathway is the C1 S-terase inhibitor. 7:43 C1 S-terase inhibitor is a protease inhibitor and inhibits factor 11A, 12A as 7:49 well as a 7:49 complement proteases, C1R and C1S. 7:53 Geneses of C1 S-terase inhibitor result in something called hereditary angiod 8:04 ema. 8:05 The final anti-thrombotic pathway involves anti-thrombin, a circulating prote 8:10 ase inhibitor. 8:11 It neutralizes most of the enzymes in the cotton cascade, inhibiting formation 8:16 of thrombin, 8:17 factor 10A and 9A. 8:22 So those were the anti-thrombotic pathways which help control and terminate the 8:26 cotton 8:26 cascade or the coagulation cascade. 8:31 Then you have fibrenolysis control mechanisms. 8:35 We already talked about thrombin, um, activatable fibrenolysis inhibitor which 8:41 slows fibrenolysis. 8:43 Another mechanism to control fibrenolysis is through plasmin activator 8:49 inhibitor PAI1, 8:51 which inhibits TPA, thus inhibiting plasmin formation. 8:56 Again another one is also alpha 2 anti-plasmin, which inhibits plasmin activity 9:02 directly. 9:04 This means that fibren is not broken down and you still have basically the clot 9:08 being 9:08 formed. 9:10 On a side note, tranexamic acid is a medication that is used to stop bleeding 9:15 by inhibiting 9:16 TPA and preventing fibrenolysis, so you want the clot to still be there. 9:21 You don't want it to break, otherwise you can bleed easily. 9:25 So thank you for watching. 9:26 In summary, we talked about the, um, the classical, uh, coagulation cascade, 9:31 which includes the 9:32 intrinsic and extrinsic pathway. 9:35 We also learned about the cell-based model, which includes initiation, 9:38 amplification and 9:39 propagation. 9:40 Finally, we talked about the control mechanisms of coagulation cascade, as well 9:45 as a control 9:46 mechanism of fibrenolysis.